The Overactivation of NADPH Oxidase during Clonorchis sinensis Infection and the Exposure to N-Nitroso Compounds Promote Periductal Fibrosis
- PMID: 34071467
- PMCID: PMC8227395
- DOI: 10.3390/antiox10060869
The Overactivation of NADPH Oxidase during Clonorchis sinensis Infection and the Exposure to N-Nitroso Compounds Promote Periductal Fibrosis
Abstract
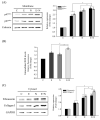
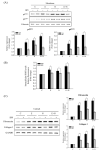
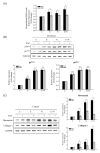
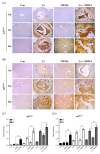
Clonorchis sinensis, a high-risk pathogenic human liver fluke, provokes various hepatobiliary complications, including epithelial hyperplasia, inflammation, periductal fibrosis, and even cholangiocarcinogenesis via direct contact with worms and their excretory-secretory products (ESPs). These pathological changes are strongly associated with persistent increases in free radical accumulation, leading to oxidative stress-mediated lesions. The present study investigated C. sinensis infection- and/or carcinogen N-nitrosodimethylamine (NDMA)-associated fibrosis in cell culture and animal models. The treatment of human cholangiocytes (H69 cells) with ESPs or/and NDMA increased reactive oxidative species (ROS) generation via the activation of NADPH oxidase (NOX), resulting in augmented expression of fibrosis-related proteins. These increased expressions were markedly attenuated by preincubation with a NOX inhibitor (diphenyleneiodonium chloride) or an antioxidant (N-acetylcysteine), indicating the involvement of excessive NOX-dependent ROS formation in periductal fibrosis. The immunoreactive NOX subunits, p47phox and p67phox, were observed in the livers of mice infected with C. sinensis and both infection plus NDMA, concomitant with collagen deposition and immunoreactive fibronectin elevation. Staining intensities are proportional to lesion severity and infection duration or/and NDMA administration. Thus, excessive ROS formation via NOX overactivation is a detrimental factor for fibrogenesis during liver fluke infection and exposure to N-nitroso compounds.
Keywords: Clonorchis sinensis infection; N-nitrosodimethylamine; NADPH oxidases; cholangiocarcinoma; cholangiocytes; excretory-secretory products; oxidative stress; periductal fibrosis.
Conflict of interest statement
The authors declare no conflict of interest in relation to this study.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

