The Immunological Role of the Placenta in SARS-CoV-2 Infection-Viral Transmission, Immune Regulation, and Lactoferrin Activity
- PMID: 34071527
- PMCID: PMC8198160
- DOI: 10.3390/ijms22115799
The Immunological Role of the Placenta in SARS-CoV-2 Infection-Viral Transmission, Immune Regulation, and Lactoferrin Activity
Abstract
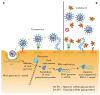
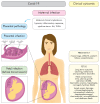
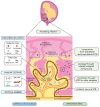
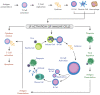
A pandemic of acute respiratory infections, due to a new type of coronavirus, can cause Severe Acute Respiratory Syndrome 2 (SARS-CoV-2) and has created the need for a better understanding of the clinical, epidemiological, and pathological features of COVID-19, especially in high-risk groups, such as pregnant women. Viral infections in pregnant women may have a much more severe course, and result in an increase in the rate of complications, including spontaneous abortion, stillbirth, and premature birth-which may cause long-term consequences in the offspring. In this review, we focus on the mother-fetal-placenta interface and its role in the potential transmission of SARS-CoV-2, including expression of viral receptors and proteases, placental pathology, and the presence of the virus in neonatal tissues and fluids. This review summarizes the current knowledge on the anti-viral activity of lactoferrin during viral infection in pregnant women, analyzes its role in the pathogenicity of pandemic virus particles, and describes the potential evidence for placental blocking/limiting of the transmission of the virus.
Keywords: COVID-19; lactoferrin; mother’s placenta; oxidative stress; pregnant women.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

