The Physical Adsorption of Gelatinized Starch with Tannic Acid Decreases the Inhibitory Activity of the Polyphenol against α-Amylase
- PMID: 34071531
- PMCID: PMC8226663
- DOI: 10.3390/foods10061233
The Physical Adsorption of Gelatinized Starch with Tannic Acid Decreases the Inhibitory Activity of the Polyphenol against α-Amylase
Abstract
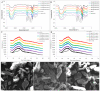
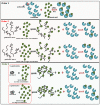
The effects of mixing orders of tannic acid (TA), starch, and α-amylase on the enzyme inhibition of TA were studied, including mixing TA with α-amylase before starch addition (order 1), mixing TA with pre-gelatinized starch before α-amylase addition (order 2) and co-gelatinizing TA with starch before α-amylase addition (order 3). It was found that the enzyme inhibition was always highest for order 1 because TA could bind with the enzyme active site thoroughly before digestion occurred. Both order 2 and 3 reduced α-amylase inhibition through decreasing binding of TA with the enzyme, which resulted from the non-covalent physical adsorption of TA with gelatinized starch. Interestingly, at low TA concentration, α-amylase inhibition for order 2 was higher than order 3, while at high TA concentration, the inhibition was shown with the opposite trend, which arose from the difference in the adsorption property between the pre-gelatinized and co-gelatinized starch at the corresponding TA concentrations. Moreover, both the crystalline structures and apparent morphology of starch were not significantly altered by TA addition for order 2 and 3. Conclusively, although a polyphenol has an acceptable inhibitory activity in vitro, the actual effect may not reach the expected one when taking processing procedures into account.
Keywords: adsorption; binding interactions; mixing order; tannic acid; α-amylase inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sun L., Wang Y., Miao M. Inhibition of α-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trends Food Sci. Technol. 2020;104:190–207. doi: 10.1016/j.tifs.2020.08.003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

