Regulation of Bone Cell Differentiation and Activation by Microbe-Associated Molecular Patterns
- PMID: 34071605
- PMCID: PMC8197933
- DOI: 10.3390/ijms22115805
Regulation of Bone Cell Differentiation and Activation by Microbe-Associated Molecular Patterns
Abstract
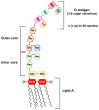
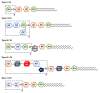
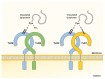
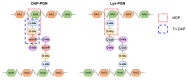
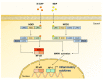
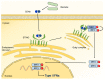
Gut microbiota has emerged as an important regulator of bone homeostasis. In particular, the modulation of innate immunity and bone homeostasis is mediated through the interaction between microbe-associated molecular patterns (MAMPs) and the host pattern recognition receptors including Toll-like receptors and nucleotide-binding oligomerization domains. Pathogenic bacteria such as Porphyromonas gingivalis and Staphylococcus aureus tend to induce bone destruction and cause various inflammatory bone diseases including periodontal diseases, osteomyelitis, and septic arthritis. On the other hand, probiotic bacteria such as Lactobacillus and Bifidobacterium species can prevent bone loss. In addition, bacterial metabolites and various secretory molecules such as short chain fatty acids and cyclic nucleotides can also affect bone homeostasis. This review focuses on the regulation of osteoclast and osteoblast by MAMPs including cell wall components and secretory microbial molecules under in vitro and in vivo conditions. MAMPs could be used as potential molecular targets for treating bone-related diseases such as osteoporosis and periodontal diseases.
Keywords: bacteria; bone diseases; bone homeostasis; microbe-associated molecular patterns; osteoblast; osteoclast; pattern-recognition receptors; secretory microbial molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Extracellular matrix networks in bone remodeling.Int J Biochem Cell Biol. 2015 Aug;65:20-31. doi: 10.1016/j.biocel.2015.05.008. Epub 2015 May 18. Int J Biochem Cell Biol. 2015. PMID: 25997875 Review.
-
Commensal Microbiota Enhance Both Osteoclast and Osteoblast Activities.Molecules. 2018 Jun 23;23(7):1517. doi: 10.3390/molecules23071517. Molecules. 2018. PMID: 29937485 Free PMC article.
-
Osteoblast-osteoclast interactions.Connect Tissue Res. 2018 Mar;59(2):99-107. doi: 10.1080/03008207.2017.1290085. Epub 2017 Mar 21. Connect Tissue Res. 2018. PMID: 28324674 Free PMC article. Review.
-
Frontline Science: Human bone cells as a source of IL-27 under inflammatory conditions: role of TLRs and cytokines.J Leukoc Biol. 2017 Jun;101(6):1289-1300. doi: 10.1189/jlb.3HI0616-280R. Epub 2016 Sep 27. J Leukoc Biol. 2017. PMID: 27677834
-
The IRF2BP2-KLF2 axis regulates osteoclast and osteoblast differentiation.BMB Rep. 2019 Jul;52(7):469-474. doi: 10.5483/BMBRep.2019.52.7.104. BMB Rep. 2019. PMID: 31186082 Free PMC article.
Cited by
-
Repair of Infected Bone Defects with Hydrogel Materials.Polymers (Basel). 2024 Jan 19;16(2):281. doi: 10.3390/polym16020281. Polymers (Basel). 2024. PMID: 38276689 Free PMC article. Review.
-
Characterization of Fecal Microbiomes of Osteoporotic Patients in Korea.Pol J Microbiol. 2022 Dec 21;71(4):601-613. doi: 10.33073/pjm-2022-045. eCollection 2022 Dec 1. Pol J Microbiol. 2022. PMID: 36537058 Free PMC article.
-
Dietary Effects of Probiotic Bacteria, Bacillus amyloliquefaciens AV5 on Growth, Serum and Mucus Immune Response, Metabolomics, and Lipid Metabolism in Nile Tilapia (Oreochromis niloticus).Aquac Nutr. 2024 Aug 12;2024:4253969. doi: 10.1155/2024/4253969. eCollection 2024. Aquac Nutr. 2024. PMID: 39555520 Free PMC article.
-
Enhancing Akkermansia growth via phytohormones: a strategy to modulate the gut-bone axis in postmenopausal osteoporosis therapy.J Transl Med. 2025 Apr 9;23(1):410. doi: 10.1186/s12967-025-06426-1. J Transl Med. 2025. PMID: 40205438 Free PMC article.
-
Effect of Bifidobacterium on osteoclasts: TNF-α/NF-κB inflammatory signal pathway-mediated mechanism.Front Endocrinol (Lausanne). 2023 Mar 9;14:1109296. doi: 10.3389/fendo.2023.1109296. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36967748 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

