Differential Antimicrobial Effect of Essential Oils and Their Main Components: Insights Based on the Cell Membrane and External Structure
- PMID: 34071618
- PMCID: PMC8227281
- DOI: 10.3390/membranes11060405
Differential Antimicrobial Effect of Essential Oils and Their Main Components: Insights Based on the Cell Membrane and External Structure
Abstract
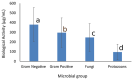
The biological activity of essential oils and their major components is well documented. Essential oils such as oregano and cinnamon are known for their effect against bacteria, fungi, and even viruses. The mechanism of action is proposed to be related to membrane and external cell structures, including cell walls. This study aimed to evaluate the biological activity of seven essential oils and eight of their major components against Gram-negative and Gram-positive bacteria, filamentous fungi, and protozoans. The antimicrobial activity was evaluated by determination of the Minimal Inhibitory Concentration for Bacillus cereus, Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, Salmonella Typhimurium, Shigella sonnei, Aspergillus niger, Aspergillus ochraceus, Alternaria alternata, and Fusarium oxysporium, the half-maximal inhibitory concentration (IC50) for Trypanosoma cruzi and Leishmania mexicana, and the median lethal dose (LD50) for Giardia lamblia. Results showed that oregano essential oil showed the best antibacterial activity (66-100 µg/mL), while cinnamon essential oil had the best fungicidal activity (66-116 µg/mL), and both showed excellent antiprotozoal activity (22-108 µg/mL). Regarding the major components, thymol and carvacrol were also good antimicrobials (23-200 µg/mL), and cinnamaldehyde was an antifungal compound (41-75 µg/mL). The major components were grouped according to their chemical structure as phenylpropanoids, terpenoids, and terpinenes. The statistical analysis of the grouped data demonstrated that protozoans were more susceptible to the essential oils, followed by fungi, Gram-positive bacteria, and Gram-negative bacteria. The analysis for the major components showed that the most resistant microbial group was fungi, which was followed by bacteria, and protozoans were also more susceptible. Principal Component Analysis for the essential oils demonstrated the relationship between the biological activity and the microbial group tested, with the first three components explaining 94.3% of the data variability. The chemical structure of the major components was also related to the biological activity presented against the microbial groups tested, where the three first principal components accounted for 91.9% of the variability. The external structures and the characteristics of the cell membranes in the different microbial groups are determinant for their susceptibility to essential oils and their major components.
Keywords: antimicrobial; cell membranes; essential oils; mechanism of action; phenylpropanoids; terpenoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms.J Agric Food Chem. 2007 May 30;55(11):4348-56. doi: 10.1021/jf063295u. Epub 2007 May 8. J Agric Food Chem. 2007. PMID: 17488023
-
Biological activity of the essential oils from Cinnamodendron dinisii and Siparuna guianensis.Braz J Microbiol. 2015 Mar 1;46(1):189-94. doi: 10.1590/S1517-838246120130683. eCollection 2015 Mar. Braz J Microbiol. 2015. PMID: 26221107 Free PMC article.
-
Chemical constituents of Helichrysum italicum (Roth) G. Don essential oil and their antimicrobial activity against Gram-positive and Gram-negative bacteria, filamentous fungi and Candida albicans.Saudi Pharm J. 2017 Jul;25(5):780-787. doi: 10.1016/j.jsps.2016.11.001. Epub 2016 Nov 12. Saudi Pharm J. 2017. PMID: 28725151 Free PMC article.
-
Essential oils: their antibacterial properties and potential applications in foods--a review.Int J Food Microbiol. 2004 Aug 1;94(3):223-53. doi: 10.1016/j.ijfoodmicro.2004.03.022. Int J Food Microbiol. 2004. PMID: 15246235 Review.
-
Potential of Plant Essential Oils and Their Components in Animal Agriculture - in vitro Studies on Antibacterial Mode of Action.Front Vet Sci. 2015 Sep 14;2:35. doi: 10.3389/fvets.2015.00035. eCollection 2015. Front Vet Sci. 2015. PMID: 26664964 Free PMC article. Review.
Cited by
-
Mexican Oregano (Lippia berlandieri Schauer and Poliomintha longiflora Gray) Essential Oils Induce Cell Death by Apoptosis in Leishmania (Leishmania) mexicana Promastigotes.Molecules. 2022 Aug 15;27(16):5183. doi: 10.3390/molecules27165183. Molecules. 2022. PMID: 36014423 Free PMC article.
-
First Report on Salvia Sahendica Boiss & Buhs roots biocomponents characterization by GC-MS and HPLC and Antibacterial Potency.Sci Rep. 2024 Oct 22;14(1):24916. doi: 10.1038/s41598-024-76679-1. Sci Rep. 2024. PMID: 39438657 Free PMC article.
-
Study of the Reinforcing Effect and Antibacterial Activity of Edible Films Based on a Mixture of Chitosan/Cassava Starch Filled with Bentonite Particles with Intercalated Ginger Essential Oil.Polymers (Basel). 2024 Sep 6;16(17):2531. doi: 10.3390/polym16172531. Polymers (Basel). 2024. PMID: 39274163 Free PMC article.
-
Synergistic Polymer Coatings with Antibacterial and Antiviral Properties for Healthcare Applications.ACS Omega. 2024 Jul 17;9(30):32662-32673. doi: 10.1021/acsomega.4c02235. eCollection 2024 Jul 30. ACS Omega. 2024. PMID: 39100336 Free PMC article.
-
Anti-Biofilms' Activity of Garlic and Thyme Essential Oils against Salmonella typhimurium.Molecules. 2022 Mar 28;27(7):2182. doi: 10.3390/molecules27072182. Molecules. 2022. PMID: 35408576 Free PMC article.
References
-
- Lappa I.K., Simini E., Nychas G.-J.E., Panagou E.Z. In vitro evaluation of essential oils against Aspergillus carbonarius isolates and their effects on Ochratoxin A related gene expression in synthetic grape medium. Food Control. 2017;73:71–80. doi: 10.1016/j.foodcont.2016.08.016. - DOI
-
- Oüzek G., Schepetkin I.A., Utegenova G.A., Kirpotina L.N., Andrei S.R., Oüzek T., Baser K.H.C., Abidkulova K.T., Kushnarenko S.V., Khlebnikov A.I., et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017;101:1361–1371. doi: 10.1189/jlb.3A1216-518RR. - DOI - PMC - PubMed
-
- Adame-Gallegos J.R., Ochoa S.A., Nevarez-Moorillon G.V. Potential Use of Mexican Oregano Essential Oil against Parasite, Fungal and Bacterial Pathogens. J. Essent. Oil Bear. Plants. 2016;19:553–567. doi: 10.1080/0972060X.2015.1116413. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

