Solid-Phase Synthesis of an Insect Pyrokinin Analog Incorporating an Imidazoline Ring as Isosteric Replacement of a trans Peptide Bond
- PMID: 34071640
- PMCID: PMC8198379
- DOI: 10.3390/molecules26113271
Solid-Phase Synthesis of an Insect Pyrokinin Analog Incorporating an Imidazoline Ring as Isosteric Replacement of a trans Peptide Bond
Abstract
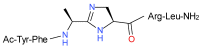
A facile solid-phase synthetic method for incorporating the imidazoline ring motif, a surrogate for a trans peptide bond, into bioactive peptides is reported. The example described is the synthesis of an imidazoline peptidomimetic analog of an insect pyrokinin neuropeptide via a cyclization reaction of an iminium salt generated from the preceding amino acid and 2,4-diaminopropanoic acid (Dap).
Keywords: SPOS; imidazoline ring; insect neuropeptides; pyrokinins; trans peptide bond.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Peptidomics of the locust corpora allata: identification of novel pyrokinins (-FXPRLamides).Peptides. 2003 Oct;24(10):1493-500. doi: 10.1016/j.peptides.2003.10.006. Peptides. 2003. PMID: 14706528
-
A brominated-fluorene insect neuropeptide analog exhibits pyrokinin/PBAN-specific toxicity for adult females of the tobacco budworm moth.Peptides. 2002 Apr;23(4):801-6. doi: 10.1016/s0196-9781(01)00656-8. Peptides. 2002. PMID: 11897401
-
Serine phosphorylation of CAPA pyrokinin in cockroaches-a taxon-specific posttranslational modification.Peptides. 2014 Jul;57:52-8. doi: 10.1016/j.peptides.2014.04.012. Epub 2014 May 2. Peptides. 2014. PMID: 24793144
-
[Molecular and physiological characterization of the pyrokinin insect neuropeptide family].Postepy Biochem. 2011;57(4):365-71. Postepy Biochem. 2011. PMID: 22568168 Review. Polish.
-
Development of amphiphylic mimics of insect neuropeptides for pest control.Ann N Y Acad Sci. 1999;897:348-60. doi: 10.1111/j.1749-6632.1999.tb07905.x. Ann N Y Acad Sci. 1999. PMID: 10676462 Review.
Cited by
-
Imidazole-amino acids. Conformational switch under tautomer and pH change.Amino Acids. 2023 Jan;55(1):33-49. doi: 10.1007/s00726-022-03201-0. Epub 2022 Nov 2. Amino Acids. 2023. PMID: 36319875 Free PMC article.
-
Advances in Research of Short Peptides.Molecules. 2022 Apr 11;27(8):2446. doi: 10.3390/molecules27082446. Molecules. 2022. PMID: 35458644 Free PMC article.
-
New Advances in Short Peptides: Looking Forward.Molecules. 2022 Jun 6;27(11):3635. doi: 10.3390/molecules27113635. Molecules. 2022. PMID: 35684571 Free PMC article.
References
-
- Jones R.C.F., Ward G.J. Amide bond isosteres: Imidazolines in pseudopeptide chemistry. Tetrahedron Lett. 1988;29:3853–3856. doi: 10.1016/S0040-4039(00)82132-2. - DOI
-
- Gilbert I.H., Rees D.C., Crocket A.K., Jones R.C.F. Imidazolines as amide bond replacements. Tetrahedron. 1995;51:6315–6336. doi: 10.1016/0040-4020(95)00273-B. - DOI
-
- Nachman R.J. Peptidomics applied: A new strategy for development of selective antagonists/agonists of insect pyrokinin (FXPRLamide) family using a novel conformational-mimetic motif. EuPA Open Proteom. 2014;3:138–142. doi: 10.1016/j.euprot.2014.02.008. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

