Development of Halogenated Pyrazolines as Selective Monoamine Oxidase-B Inhibitors: Deciphering via Molecular Dynamics Approach
- PMID: 34071665
- PMCID: PMC8198649
- DOI: 10.3390/molecules26113264
Development of Halogenated Pyrazolines as Selective Monoamine Oxidase-B Inhibitors: Deciphering via Molecular Dynamics Approach
Abstract
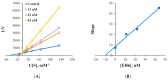
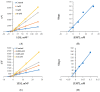
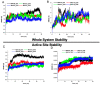
Halogens have been reported to play a major role in the inhibition of monoamine oxidase (MAO), relating to diverse cognitive functions of the central nervous system. Pyrazoline/halogenated pyrazolines were investigated for their inhibitory activities against human monoamine oxidase-A and -B. Halogen substitutions on the phenyl ring located at the fifth position of pyrazoline showed potent MAO-B inhibition. Compound 3-(4-ethoxyphenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazole (EH7) showed the highest potency against MAO-B with an IC50 value of 0.063 µM. The potencies against MAO-B were increased in the order of -F (in EH7) > -Cl (EH6) > -Br (EH8) > -H (EH1). The residual activities of most compounds for MAO-A were > 50% at 10 µM, except for EH7 and EH8 (IC50 = 8.38 and 4.31 µM, respectively). EH7 showed the highest selectivity index (SI) value of 133.0 for MAO-B, followed by EH6 at > 55.8. EH7 was a reversible and competitive inhibitor of MAO-B in kinetic and reversibility experiments with a Ki value of 0.034 ± 0.0067 µM. The molecular dynamics study documented that EH7 had a good binding affinity and motional movement within the active site with high stability. It was observed by MM-PBSA that the chirality had little effect on the overall binding of EH7 to MAO-B. Thus, EH7 can be employed for the development of lead molecules for the treatment of various neurodegenerative disorders.
Keywords: halogenated pyrazolines; kinetics; molecular dynamics; monoamine oxidase inhibitors; reversibility.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

