Cefiderocol in Critically Ill Patients with Multi-Drug Resistant Pathogens: Real-Life Data on Pharmacokinetics and Microbiological Surveillance
- PMID: 34071700
- PMCID: PMC8226704
- DOI: 10.3390/antibiotics10060649
Cefiderocol in Critically Ill Patients with Multi-Drug Resistant Pathogens: Real-Life Data on Pharmacokinetics and Microbiological Surveillance
Erratum in
-
Correction: König et al. Cefiderocol in Critically Ill Patients with Multi-Drug Resistant Pathogens: Real-Life Data on Pharmacokinetics and Microbiological Surveillance. Antibiotics 2021, 10, 649.Antibiotics (Basel). 2021 Oct 9;10(10):1230. doi: 10.3390/antibiotics10101230. Antibiotics (Basel). 2021. PMID: 34680855 Free PMC article.
Abstract
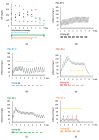
Cefiderocol is a new siderophore-cephalosporin for the treatment of multi-drug resistant Gram-negative pathogens. As a reserve agent, it will and should be used primarily in critically ill patients in the upcoming years. Due to the novelty of the substance little data on the pharmacokinetics in critically ill patients with septic shock and renal failure (including continuous renal replacement therapy and cytokine adsorber therapy) is available. We performed therapeutic drug monitoring in a cohort of five patients treated with cefiderocol, to improve the knowledge on pharmacokinetics in this vulnerable patient population. As expected for a cephalosporin with predominantly renal elimination the maintenance dose could be reduced in patients with renal impairment or on continuous renal replacement therapy. The manufacturer's dosing instructions were sufficient to achieve a drug level well above the MIC. However, the addition of a cytokine adsorber might reduce serum levels substantially, so that in this context therapeutic drug monitoring and dose adjustment are recommended.
Keywords: cefiderocol; continuous veno-venous hemodialysis; critically ill patients; pharmacokinetics; therapeutic drug monitoring.
Conflict of interest statement
C. König reports lecture fees from AMEOS; H. Rohde reports personal consultant and lecture fees from Correvio, Infectopharm, INSMED, MSD, Pfizer and Shionoogi; D. Wichmann reports personal consultant and lecture fees from AMEOS, Correvio, Eumedica, EUSA, Gilead, Kite, MSD, Novartis, Pfizer and Shionogi. A. Both, A. C. Röhr and O. R. Frey report no conflict of interest related to this work.
Figures
References
-
- Bassetti M., Echols R., Matsunaga Y., Ariyasu M., Doi Y., Ferrer R., Lodise T.P., Naas T., Niki Y., Paterson D.L., et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021;21:226–240. doi: 10.1016/S1473-3099(20)30796-9. - DOI - PubMed
-
- Wunderink R.G., Matsunaga Y., Ariyasu M., Clevenbergh P., Echols R., Kaye K.S., Kollef M., Menon A., Pogue J.M., Shorr A.F., et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021;21:213–225. doi: 10.1016/S1473-3099(20)30731-3. - DOI - PubMed
-
- Kazmierczak K.M., Tsuji M., Wise M.G., Hackel M., Yamano Y., Echols R., Sahm D.F. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study) Int. J. Antimicrob. Agents. 2019;53:177–184. doi: 10.1016/j.ijantimicag.2018.10.007. - DOI - PubMed
-
- Ito A., Kohira N., Bouchillon S.K., West J., Rittenhouse S., Sader H.S., Rhomberg P.R., Jones R.N., Yoshizawa H., Nakamura R., et al. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J. Antimicrob. Chemother. 2016;71:670–677. doi: 10.1093/jac/dkv402. - DOI - PubMed
-
- Zhanel G.G., Golden A.R., Zelenitsky S., Wiebe K., Lawrence C.K., Adam H.J., Idowu T., Domalaon R., Schweizer F., Zhanel M.A., et al. Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs. 2019;79:271–289. doi: 10.1007/s40265-019-1055-2. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

