Strongyloides-Specific IgE Phage cDNA Clones and Development of a Novel ELISA for Strongyloidiasis
- PMID: 34071716
- PMCID: PMC8228214
- DOI: 10.3390/diagnostics11060985
Strongyloides-Specific IgE Phage cDNA Clones and Development of a Novel ELISA for Strongyloidiasis
Abstract
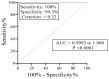
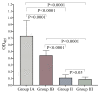
Strongyloidiasis, caused mainly by the nematode Strongyloides stercoralis, is prevalent worldwide and potentially fatal in immunosuppressed patients. We report on a new IgE biomarker to diagnose Strongyloides infection. Sera from two groups infected with Strongyloides served as positive samples: Group 1A, in which infection was confirmed by stool-microscopy and/or stool-polymerase chain reaction (PCR) and was seropositive by an IgG-enzyme linked immunosorbent assay (ELISA) and an IgG4 rapid test, and Group 1B in which infection was confirmed by stool-PCR but was seronegative. Negative samples (controls) comprised infections with other parasites (Group II) and healthy donors (Group III). Immunoscreenings of an S. stercoralis complementary DNA (cDNA) library were performed, and the cDNA clone with the highest diagnostic potential (clone A133) was selected for recombinant protein production and then evaluated using IgE Western blot and ELISA. The Western blot showed that the recombinant protein (rA133) was 100% reactive with Group IA (n = 10) and Group IB (n = 5), and 96% non-reactive with Groups II and III (n = 25). Subsequently, the IgE-ELISA was developed and showed 100% diagnostic sensitivity in Groups IA (n = 32) and IB (n = 11); and 99.3% specificity in Groups II and III (n = 144). In conclusion, this study has identified rA133 as a novel recombinant protein with potential diagnostic value, and that the IgE-ELISA incorporating this protein may be useful for patient diagnosis and epidemiological studies.
Keywords: IgE-enzyme linked immunosorbent assay (ELISA); Strongyloides stercoralis; complementary DNA (cDNA) library; immunoscreening; recombinant antigen; serodiagnosis; strongyloidiasis.
Conflict of interest statement
R.N., H.A., and N.A. are named as inventors in a patent on the use of rA133 for diagnosis of strongyloidiasis, which has been filed in Malaysia (PI 2020000313) and PCT (PCT/MY2020/050044).
Figures


Similar articles
-
Diagnostic Potential of an IgE-ELISA in Detecting Strongyloidiasis.Am J Trop Med Hyg. 2020 Dec;103(6):2288-2293. doi: 10.4269/ajtmh.20-0265. Epub 2020 Sep 24. Am J Trop Med Hyg. 2020. PMID: 32996454 Free PMC article.
-
IgG1, IgG4, and IgE antibody responses in human strongyloidiasis by ELISA using Strongyloides ratti saline extract as heterologous antigen.Parasitol Res. 2007 Oct;101(5):1209-14. doi: 10.1007/s00436-007-0602-z. Epub 2007 Jul 4. Parasitol Res. 2007. PMID: 17610082
-
A new antigen detection ELISA for the diagnosis of Strongyloides infection.Acta Trop. 2021 Sep;221:105986. doi: 10.1016/j.actatropica.2021.105986. Epub 2021 May 28. Acta Trop. 2021. PMID: 34058161
-
Serodiagnosis and early detection of Strongyloides stercoralis infection.J Microbiol Immunol Infect. 2019 Jun;52(3):371-378. doi: 10.1016/j.jmii.2018.10.001. Epub 2018 Oct 11. J Microbiol Immunol Infect. 2019. PMID: 30482708 Review.
-
Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis.Parasite Immunol. 2004 Nov-Dec;26(11-12):487-97. doi: 10.1111/j.0141-9838.2004.00726.x. Parasite Immunol. 2004. PMID: 15771684 Review.
Cited by
-
Are humanized IgE reporter systems potential game changers in serological diagnosis of human parasitic infection?Parasitol Res. 2022 Apr;121(4):1137-1144. doi: 10.1007/s00436-021-07352-z. Epub 2021 Nov 12. Parasitol Res. 2022. PMID: 34767081 Free PMC article.
-
Systematic Review of Strongyloides stercoralis Infection Diagnosis in Southeast Asia: Insights from Parasitological, Molecular, and Serological Approaches.Am J Trop Med Hyg. 2024 Aug 13;111(4):724-735. doi: 10.4269/ajtmh.23-0599. Print 2024 Oct 2. Am J Trop Med Hyg. 2024. PMID: 39137756
-
Serological diagnosis of cysticercosis in humans and pigs: status, limitations, and prospects.Front Vet Sci. 2025 Jul 15;12:1558555. doi: 10.3389/fvets.2025.1558555. eCollection 2025. Front Vet Sci. 2025. PMID: 40735286 Free PMC article. Review.
-
Design and expression of a chimeric recombinant antigen (SsIR-Ss1a) for the serodiagnosis of human strongyloidiasis: Evaluation of performance, sensitivity, and specificity.PLoS Negl Trop Dis. 2024 Jul 15;18(7):e0012320. doi: 10.1371/journal.pntd.0012320. eCollection 2024 Jul. PLoS Negl Trop Dis. 2024. PMID: 39008519 Free PMC article.
References
-
- Schad G.A. Morphology and life history of Strongyloides stercoralis. In: Grove D.I., editor. Strongyloidiasis a Major Roundworm Infection of Man. Taylor & Francis; London, UK: 1989. pp. 85–104.
-
- Thanchomnang T., Intapan P.M., Sanpool O., Rodpai R., Tourtip S., Yahom S., Kullawat J., Radomyos P., Thammasiri C., Maleewong W. First molecular identification and genetic diversity of Strongyloides stercoralis and Strongyloides fuelleborni in human communities having contact with long-tailed macaques in Thailand. Parasitol. Res. 2017;116:1917–1923. doi: 10.1007/s00436-017-5469-z. - DOI - PubMed
-
- Sheild J.M., Kow F., Shield J.M., Kow F. A comparative study of intestinal helminths in pre-school age urban and rural children in Morobe Province, Papua New Guinea. Papua New Guinea Med. J. 2013;56:14–31. - PubMed
Grants and funding
- R01 AI050668/AI/NIAID NIH HHS/United States
- R21 AI144572/AI/NIAID NIH HHS/United States
- FRGS/1/2018/SKK08/USM/01/2; Acc. No. 203.CIPPM.6711636/Malaysian Ministry of Higher Education (MOHE) FRGS grant
- No: 311/CIPPM/4401005/MOHE Higher Institution Centre of Excellence (HICoE) program
- Grant AI050668/US National Institutes of Health
LinkOut - more resources
Full Text Sources

