Sensitivity to Haptic Sound-Localization Cues at Different Body Locations
- PMID: 34071729
- PMCID: PMC8198414
- DOI: 10.3390/s21113770
Sensitivity to Haptic Sound-Localization Cues at Different Body Locations
Abstract
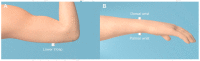
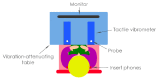
Cochlear implants (CIs) recover hearing in severely to profoundly hearing-impaired people by electrically stimulating the cochlea. While they are extremely effective, spatial hearing is typically severely limited. Recent studies have shown that haptic stimulation can supplement the electrical CI signal (electro-haptic stimulation) and substantially improve sound localization. In haptic sound-localization studies, the signal is extracted from the audio received by behind-the-ear devices and delivered to each wrist. Localization is achieved using tactile intensity differences (TIDs) across the wrists, which match sound intensity differences across the ears (a key sound localization cue). The current study established sensitivity to across-limb TIDs at three candidate locations for a wearable haptic device, namely: the lower tricep and the palmar and dorsal wrist. At all locations, TID sensitivity was similar to the sensitivity to across-ear intensity differences for normal-hearing listeners. This suggests that greater haptic sound-localization accuracy than previously shown can be achieved. The dynamic range was also measured and far exceeded that available through electrical CI stimulation for all of the locations, suggesting that haptic stimulation could provide additional sound-intensity information. These results indicate that an effective haptic aid could be deployed for any of the candidate locations, and could offer a low-cost, non-invasive means of improving outcomes for hearing-impaired listeners.
Keywords: cochlear implant; cross-modal; electro-haptic stimulation; haptic sound-localization; hearing aid; hearing impaired; neuroprosthetic; somatosensory; tactile; vibrotactile.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Can Haptic Stimulation Enhance Music Perception in Hearing-Impaired Listeners?Front Neurosci. 2021 Aug 31;15:723877. doi: 10.3389/fnins.2021.723877. eCollection 2021. Front Neurosci. 2021. PMID: 34531717 Free PMC article. Review.
-
Electro-Haptic Enhancement of Spatial Hearing in Cochlear Implant Users.Sci Rep. 2020 Jan 31;10(1):1621. doi: 10.1038/s41598-020-58503-8. Sci Rep. 2020. PMID: 32005889 Free PMC article.
-
Sensitivity to haptic sound-localisation cues.Sci Rep. 2021 Jan 11;11(1):312. doi: 10.1038/s41598-020-79150-z. Sci Rep. 2021. PMID: 33431929 Free PMC article.
-
Using haptic stimulation to enhance auditory perception in hearing-impaired listeners.Expert Rev Med Devices. 2021 Jan;18(1):63-74. doi: 10.1080/17434440.2021.1863782. Epub 2020 Dec 29. Expert Rev Med Devices. 2021. PMID: 33372550 Review.
-
Haptic sound-localisation for use in cochlear implant and hearing-aid users.Sci Rep. 2020 Aug 25;10(1):14171. doi: 10.1038/s41598-020-70379-2. Sci Rep. 2020. PMID: 32843659 Free PMC article. Clinical Trial.
Cited by
-
Improved tactile speech perception and noise robustness using audio-to-tactile sensory substitution with amplitude envelope expansion.Sci Rep. 2024 Jul 1;14(1):15029. doi: 10.1038/s41598-024-65510-6. Sci Rep. 2024. PMID: 38951556 Free PMC article.
-
Can Haptic Stimulation Enhance Music Perception in Hearing-Impaired Listeners?Front Neurosci. 2021 Aug 31;15:723877. doi: 10.3389/fnins.2021.723877. eCollection 2021. Front Neurosci. 2021. PMID: 34531717 Free PMC article. Review.
-
Electro-Haptic Stimulation: A New Approach for Improving Cochlear-Implant Listening.Front Neurosci. 2021 Jun 9;15:581414. doi: 10.3389/fnins.2021.581414. eCollection 2021. Front Neurosci. 2021. PMID: 34177440 Free PMC article. Review.
-
Improved tactile speech perception using audio-to-tactile sensory substitution with formant frequency focusing.Sci Rep. 2024 Feb 28;14(1):4889. doi: 10.1038/s41598-024-55429-3. Sci Rep. 2024. PMID: 38418558 Free PMC article.
-
Improving speech perception for hearing-impaired listeners using audio-to-tactile sensory substitution with multiple frequency channels.Sci Rep. 2023 Aug 16;13(1):13336. doi: 10.1038/s41598-023-40509-7. Sci Rep. 2023. PMID: 37587166 Free PMC article.
References
-
- Spriet A., Van Deun L., Eftaxiadis K., Laneau J., Moonen M., van Dijk B., van Wieringen A., Wouters J. Speech understanding in background noise with the two-microphone adaptive beamformer BEAM™ in the Nucleus Freedom™ cochlear implant system. Ear Hear. 2007;28:62–72. doi: 10.1097/01.aud.0000252470.54246.54. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

