Phospholipid-Conjugated PEG- b-PCL Copolymers as Precursors of Micellar Vehicles for Amphotericin B
- PMID: 34071785
- PMCID: PMC8199447
- DOI: 10.3390/polym13111747
Phospholipid-Conjugated PEG- b-PCL Copolymers as Precursors of Micellar Vehicles for Amphotericin B
Abstract
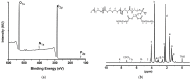
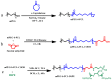
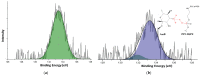
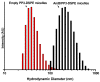
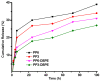
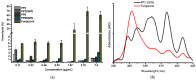
Amphotericin B (AmB) is a widely used antifungal that presents a broad action spectrum and few reports on the development of resistance. However, AmB is highly toxic, causing renal failure in a considerable number of treated patients. Although when AmB is transported via polymer micelles (PMs) as delivery vehicles its nephrotoxicity has been successfully attenuated, this type of nanoparticle has limitations, such as low encapsulation capacity and poor stability in aqueous media. In this research, the effect of modifying polyethyleglicol-block-poly(ε-caprolactone) (PEG-b-PCL) with 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE) on the performance of PMs as vehicles for AmB was studied. PEG-b-PCL with two different lengths of a PCL segment was prepared via ring opening polymerisation and modified with DSPE at a post-synthesis stage through amidation. Upon modification with DSPE, a copolymer was self-assembled, thereby producing particles with hydrodynamic diameters below 100 nm and a lower critical micelle concentration than that of the raw copolymers. Likewise, in the presence of DSPE, the loading capacity of AmB increased because of the formed intermolecular interactions, such as hydrogen bonds, which also caused a lower aggregation of this drug. The assessment of in vitro toxicity against red blood cells indicated that the toxicity of AmB decreased upon encapsulation; however, its antifungal action against clinical yeasts was maintained and enhanced, as indicated by a decrease in its minimum inhibitory concentration.
Keywords: amphotericin B; phospholipid-modified copolymer; polymer micelle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Singh P.K., Gorain B., Choudhury H., Singh S.K., Whadwa P., Sahu S., Gulati M., Kesharwani P. Macro-phage targeted amphotericin B nanodelivery systems against visceral leishmaniasis. Mater. Sci. Eng. B. 2020;258:114571. doi: 10.1016/j.mseb.2020.114571. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

