Asymmetric Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives
- PMID: 34071844
- PMCID: PMC8199250
- DOI: 10.3390/molecules26113202
Asymmetric Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives
Abstract
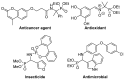
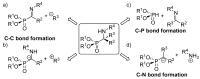
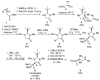
Due to their structural similarity with natural α-amino acids, α-aminophosphonic acid derivatives are known biologically active molecules. In view of the relevance of tetrasubstituted carbons in nature and medicine and the strong dependence of the biological activity of chiral molecules into their absolute configuration, the synthesis of α-aminophosphonates bearing tetrasubstituted carbons in an asymmetric fashion has grown in interest in the past few decades. In the following lines, the existing literatures for the synthesis of optically active tetrasubstituted α-aminophosphonates are summarized, comprising diastereoselective and enantioselective approaches.
Keywords: asymmetric synthesis; diastereoselective; enantioselective; tetrasubstituted carbons; α-aminophosphonates; α-aminophosphonic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

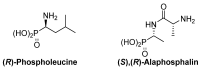



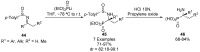
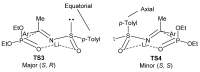
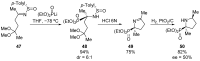


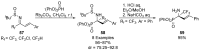






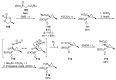



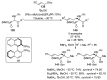


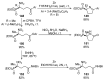

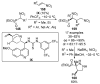



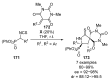

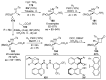

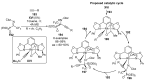



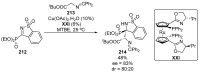





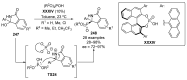



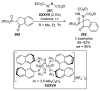

References
-
- Duthaler R.O. Recent developments in the stereoselective synthesis of α-aminoacids. Tetrahedron. 1994;50:1539–1650. doi: 10.1016/S0040-4020(01)80840-1. - DOI
-
- Berlicki L., Kafarski P. Computer-Aided Analysis and Design of Phosphonic and Phosphinic Enzyme Inhibitors as Potential Drugs and Agrochemicals. Curr. Org. Chem. 2005;9:1829–1850. doi: 10.2174/138527205774913088. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

