Carbazole-Based Colorimetric Anion Sensors
- PMID: 34071969
- PMCID: PMC8199442
- DOI: 10.3390/molecules26113205
Carbazole-Based Colorimetric Anion Sensors
Abstract
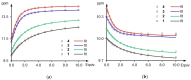
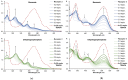
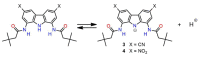
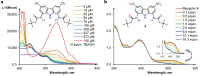
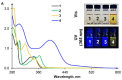
Owing to their strong carbazole chromophore and fluorophore, as well as to their powerful and convergent hydrogen bond donors, 1,8-diaminocarbazoles are amongst the most attractive and synthetically versatile building blocks for the construction of anion receptors, sensors, and transporters. Aiming to develop carbazole-based colorimetric anion sensors, herein we describe the synthesis of 1,8-diaminocarbazoles substituted with strongly electron-withdrawing substituents, i.e., 3,6-dicyano and 3,6-dinitro. Both of these precursors were subsequently converted into model diamide receptors. Anion binding studies revealed that the new receptors exhibited significantly enhanced anion affinities, but also significantly increased acidities. We also found that rear substitution of 1,8-diamidocarbazole with two nitro groups shifted its absorption spectrum into the visible region and converted the receptor into a colorimetric anion sensor. The new sensor displayed vivid color and fluorescence changes upon addition of basic anions in wet dimethyl sulfoxide, but it was poorly selective; because of its enhanced acidity, the dominant receptor-anion interaction for most anions was proton transfer and, accordingly, similar changes in color were observed for all basic anions. The highly acidic and strongly binding receptors developed in this study may be applicable in organocatalysis or in pH-switchable anion transport through lipophilic membranes.
Keywords: anion receptors; anion recognition; anion sensors; colorimetric sensors; supramolecular chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




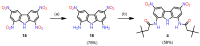



Similar articles
-
A new generation of 1,8-diaminocarbazole building blocks for the construction of fluorescent anion receptors.RSC Adv. 2024 Sep 19;14(41):29883-29889. doi: 10.1039/d4ra05420b. eCollection 2024 Sep 18. RSC Adv. 2024. PMID: 39301241 Free PMC article.
-
1,8-Diamidocarbazoles: an easily tuneable family of fluorescent anion sensors and transporters.Org Biomol Chem. 2018 Jul 18;16(28):5188-5196. doi: 10.1039/c8ob01031e. Org Biomol Chem. 2018. PMID: 29971303
-
Boosting Anion Transport Activity of Diamidocarbazoles by Electron Withdrawing Substituents.Front Chem. 2021 May 20;9:690035. doi: 10.3389/fchem.2021.690035. eCollection 2021. Front Chem. 2021. PMID: 34095089 Free PMC article.
-
Steroid-based anion receptors and transporters.Chem Soc Rev. 2010 Oct;39(10):3633-47. doi: 10.1039/b926225n. Epub 2010 Aug 11. Chem Soc Rev. 2010. PMID: 20714471 Review.
-
Colorimetric and fluorescent anion sensors: an overview of recent developments in the use of 1,8-naphthalimide-based chemosensors.Chem Soc Rev. 2010 Oct;39(10):3936-53. doi: 10.1039/b910560n. Epub 2010 Sep 6. Chem Soc Rev. 2010. PMID: 20818454 Review.
Cited by
-
Carbazole Framework as Functional Scaffold for the Design of Synthetic Receptors.Chemistry. 2025 May 27;31(30):e202500126. doi: 10.1002/chem.202500126. Epub 2025 Apr 3. Chemistry. 2025. PMID: 40101001 Free PMC article. Review.
-
A new generation of 1,8-diaminocarbazole building blocks for the construction of fluorescent anion receptors.RSC Adv. 2024 Sep 19;14(41):29883-29889. doi: 10.1039/d4ra05420b. eCollection 2024 Sep 18. RSC Adv. 2024. PMID: 39301241 Free PMC article.
References
-
- Al-Sayah M.H., Abdalla A.M., Shehab M.K. A dansyl-based optical probe for detection of singly and doubly charged anions. Supramol. Chem. 2016;28:224–230. doi: 10.1080/10610278.2015.1091456. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

