Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic
- PMID: 34071979
- PMCID: PMC8229489
- DOI: 10.3390/cells10061327
Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic
Abstract
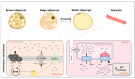
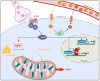
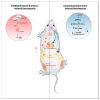
Thyroid hormones (TH) contribute to the control of adaptive thermogenesis, which is associated with both higher energy expenditure and lower body mass index. While it was clearly established that TH act directly in the target tissues to fulfill its metabolic activities, some studies have rather suggested that TH act in the hypothalamus to control these processes. This paradigm shift has subjected the topic to intense debates. This review aims to recapitulate how TH control adaptive thermogenesis and to what extent the brain is involved in this process. This is of crucial importance for the design of new pharmacological agents that would take advantage of the TH metabolic properties.
Keywords: brown adipose tissue; browning; hypothalamus; thermogenesis; thyroid hormones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Thyroid hormones and browning of adipose tissue.Mol Cell Endocrinol. 2017 Dec 15;458:156-159. doi: 10.1016/j.mce.2017.01.011. Epub 2017 Jan 12. Mol Cell Endocrinol. 2017. PMID: 28089823 Review.
-
Novel Aspects of White Adipose Tissue Browning by Thyroid Hormones.Exp Clin Endocrinol Diabetes. 2020 Jun;128(6-07):446-449. doi: 10.1055/a-1020-5354. Epub 2019 Nov 7. Exp Clin Endocrinol Diabetes. 2020. PMID: 31698480 Review.
-
Thermogenesis in Adipose Tissue Activated by Thyroid Hormone.Int J Mol Sci. 2020 Apr 24;21(8):3020. doi: 10.3390/ijms21083020. Int J Mol Sci. 2020. PMID: 32344721 Free PMC article. Review.
-
Variable Cold-Induced Brown Adipose Tissue Response to Thyroid Hormone Status.Thyroid. 2017 Jan;27(1):1-10. doi: 10.1089/thy.2015.0646. Epub 2016 Nov 29. Thyroid. 2017. PMID: 27750020 Free PMC article.
-
ADGRA1 negatively regulates energy expenditure and thermogenesis through both sympathetic nervous system and hypothalamus-pituitary-thyroid axis in male mice.Cell Death Dis. 2021 Apr 6;12(4):362. doi: 10.1038/s41419-021-03634-7. Cell Death Dis. 2021. PMID: 33824276 Free PMC article.
Cited by
-
The ecological function of thyroid hormones.Philos Trans R Soc Lond B Biol Sci. 2024 Mar 25;379(1898):20220511. doi: 10.1098/rstb.2022.0511. Epub 2024 Feb 5. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38310932 Free PMC article. Review.
-
Temperature Differences Between Controlled Primary Hypothyroidism and Healthy Patients: An Exploratory Study.J Endocr Soc. 2024 Jan 2;8(2):bvad175. doi: 10.1210/jendso/bvad175. eCollection 2024 Jan 5. J Endocr Soc. 2024. PMID: 38196662 Free PMC article.
-
Comparative analysis of thyroid hormone systems in rodents with subterranean lifestyle.Sci Rep. 2023 Feb 22;13(1):3122. doi: 10.1038/s41598-023-30179-w. Sci Rep. 2023. PMID: 36813840 Free PMC article.
-
Strategies for Hypothermia Compensation in Altricial and Precocial Newborn Mammals and Their Monitoring by Infrared Thermography.Vet Sci. 2022 May 23;9(5):246. doi: 10.3390/vetsci9050246. Vet Sci. 2022. PMID: 35622774 Free PMC article. Review.
-
Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets.Signal Transduct Target Ther. 2024 Apr 26;9(1):97. doi: 10.1038/s41392-024-01811-6. Signal Transduct Target Ther. 2024. PMID: 38664391 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

