Functional Characterization of the Obesity-Linked Variant of the β3-Adrenergic Receptor
- PMID: 34072007
- PMCID: PMC8199065
- DOI: 10.3390/ijms22115721
Functional Characterization of the Obesity-Linked Variant of the β3-Adrenergic Receptor
Abstract
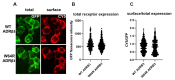
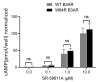
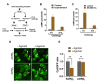
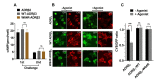
Adrenergic receptor β3 (ADRβ3) is a member of the rhodopsin-like G protein-coupled receptor family. The binding of the ligand to ADRβ3 activates adenylate cyclase and increases cAMP in the cells. ADRβ3 is highly expressed in white and brown adipocytes and controls key regulatory pathways of lipid metabolism. Trp64Arg (W64R) polymorphism in the ADRβ3 is associated with the early development of type 2 diabetes mellitus, lower resting metabolic rate, abdominal obesity, and insulin resistance. It is unclear how the substitution of W64R affects the functioning of ADRβ3. This study was initiated to functionally characterize this obesity-linked variant of ADRβ3. We evaluated in detail the expression, subcellular distribution, and post-activation behavior of the WT and W64R ADRβ3 using single cell quantitative fluorescence microscopy. When expressed in HEK 293 cells, ADRβ3 shows a typical distribution displayed by other GPCRs with a predominant localization at the cell surface. Unlike adrenergic receptor β2 (ADRβ2), agonist-induced desensitization of ADRβ3 does not involve loss of cell surface expression. WT and W64R variant of ADRβ3 displayed comparable biochemical properties, and there was no significant impact of the substitution of tryptophan with arginine on the expression, cellular distribution, signaling, and post-activation behavior of ADRβ3. The obesity-linked W64R variant of ADRβ3 is indistinguishable from the WT ADRβ3 in terms of expression, cellular distribution, signaling, and post-activation behavior.
Keywords: G-protein coupled receptors; beta-3-adrenergic receptor; receptor desensitization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The beta3-adrenergic receptor in the obesity and diabetes prone rhesus monkey is very similar to human and contains arginine at codon 64.Gene. 1997 Apr 1;188(2):207-13. doi: 10.1016/s0378-1119(96)00796-2. Gene. 1997. PMID: 9133593
-
Interactions between variants in the beta3-adrenergic receptor and peroxisome proliferator-activated receptor-gamma2 genes and obesity.Diabetes Care. 2001 Apr;24(4):672-7. doi: 10.2337/diacare.24.4.672. Diabetes Care. 2001. PMID: 11315829
-
Rapid genotyping using real-time fluorescent PCR of the Trp64Arg polymorphism of the beta3-adrenergic receptor gene and the -3826 A to G variant of the uncoupling protein-1 gene.Biochem Genet. 2007 Dec;45(11-12):769-73. doi: 10.1007/s10528-007-9116-8. Epub 2007 Nov 27. Biochem Genet. 2007. PMID: 18040773 No abstract available.
-
[Beta3-adrenergic receptor].Postepy Biochem. 2005;51(1):80-7. Postepy Biochem. 2005. PMID: 16209345 Review. Polish.
-
[Beta3-adrenergic receptor gene polymorphism in diabetes].Nihon Rinsho. 2005 Feb;63 Suppl 2:180-4. Nihon Rinsho. 2005. PMID: 15779366 Review. Japanese. No abstract available.
Cited by
-
Play the plug: How bacteria modify recognition by host receptors?Front Microbiol. 2022 Oct 14;13:960326. doi: 10.3389/fmicb.2022.960326. eCollection 2022. Front Microbiol. 2022. PMID: 36312954 Free PMC article.
-
Effects of Genetic Mutation Sites in ADR Genes on Modern Chickens Produced and Domesticated by Artificial Selection.Biology (Basel). 2023 Jan 20;12(2):169. doi: 10.3390/biology12020169. Biology (Basel). 2023. PMID: 36829448 Free PMC article.
-
The β3-Adrenergic Receptor: Structure, Physiopathology of Disease, and Emerging Therapeutic Potential.Adv Pharmacol Pharm Sci. 2024 Nov 28;2024:2005589. doi: 10.1155/2024/2005589. eCollection 2024. Adv Pharmacol Pharm Sci. 2024. PMID: 39640497 Free PMC article. Review.
-
Stress regulatory hormones and cancer: the contribution of epinephrine and cancer therapeutic value of beta blockers.Endocrine. 2025 May;88(2):359-386. doi: 10.1007/s12020-025-04161-7. Epub 2025 Jan 27. Endocrine. 2025. PMID: 39869294 Review.
-
Special Issue: "G Protein-Coupled Receptor and Their Kinases in Cell Biology and Disease 2.0".Int J Mol Sci. 2022 Dec 2;23(23):15152. doi: 10.3390/ijms232315152. Int J Mol Sci. 2022. PMID: 36499478 Free PMC article.
References
-
- Aurbach G.D., Spiegel A.M., Gardner J.D. Beta-adrenergic receptors, cyclic AMP, and ion transport in the avian erythrocyte. Adv. Cycl. Nucleotide Res. 1975;5:117–132. - PubMed
-
- Robinson G., Sutherland E.W. On the relation of cyclic AMP to adrenergic receptors and sympathin. Adv. Cytopharmacol. 1971;1:263–272. - PubMed
-
- Femminella G.D., Rengo G., Pagano G., de Lucia C., Komici K., Parisi V., Cannavo A., Liccardo D., Vigorito C., Filardi P.P., et al. Beta-adrenergic receptors and G protein-coupled receptor kinase-2 in Alzheimer’s disease: A new paradigm for prognosis and therapy? J. Alzheimers Dis. 2013;34:341–347. doi: 10.3233/JAD-121813. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

