Virus-Associated Biomarkers in Oropharyngeal and Nasopharyngeal Cancers and Recurrent Respiratory Papillomatosis
- PMID: 34072019
- PMCID: PMC8228279
- DOI: 10.3390/microorganisms9061150
Virus-Associated Biomarkers in Oropharyngeal and Nasopharyngeal Cancers and Recurrent Respiratory Papillomatosis
Abstract
Nasopharyngeal cancer (NPC) is known to be associated with Epstein-Barr virus (EBV). Pre-treatment and post-treatment detection of plasma cell-free EBV DNA has been shown to be useful as a diagnostic as well as a prognostic factor in NPC. On the other hand, the incidence of human papillomavirus (HPV)-associated oropharyngeal cancer (OPC) is increasing. In contrast to cervical cancer, which is classically known to be an HPV-associated malignancy, HPV testing is not clinically applied for OPC, except for p16 immunostaining as a surrogate marker of HPV infection. One of the major characteristics of HPV-associated OPC is its association with a good prognosis compared with non-HPV-associated OPC. However, some patients still have a poor prognosis. Another characteristic of HPV-associated OPC is the distinct risk factor of high sexual activity. Establishing a biomarker for the prediction of the prognosis before and/or after initial treatment, as well as for diagnosis in populations at high risk, is of marked interest. With this background, HPV DNA detection in plasma and oral rinses has become an area of focus. In this review, the current significance of HPV DNA detection in plasma and oral rinse samples, as well as serum HPV antibody levels, is evaluated.
Keywords: DNA; biomarker; human papillomavirus; oral rinse; oropharyngeal cancer; plasma; recurrent respiratory papillomatosis.
Conflict of interest statement
The author declares no conflict of interest.
Figures
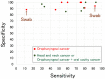
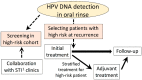
References
-
- Kondo S., Horikawa T., Takeshita H., Kanegane C., Kasahara Y., Sheen T., Sato H., Furukawa M., Yoshizaki T. Diagnostic value of serum EBV-DNA quantification and antibody to viral capsid antigen in nasopharyngeal carcinoma patients. Cancer Sci. 2004;85:508–513. doi: 10.1111/j.1349-7006.2004.tb03241.x. - DOI - PMC - PubMed
-
- Lo Y.M., Chan L.Y., Lo K.W., Leung S.F., Zhang J., Chan A.T., Lee J.C., Hjelm N.M., Johnson P.J., Huang D.P. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999;59:1188–1191. - PubMed
-
- Zhang W., Chen T., Chen L., Guo R., Zhou G., Tang L., Mao Y., Li W., Liu X., Du X., et al. The clinical utility of plasma Epstein-Barr virus DNA assays in nasopharyngeal carcinoma: The dawn of a new era? A systematic review and meta-analysis of 7836 cases. Medicine. 2015;94:e845. doi: 10.1097/MD.0000000000000845. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

