Pharmacological Blockade of PPARα Exacerbates Inflammatory Pain-Related Impairment of Spatial Memory in Rats
- PMID: 34072060
- PMCID: PMC8227714
- DOI: 10.3390/biomedicines9060610
Pharmacological Blockade of PPARα Exacerbates Inflammatory Pain-Related Impairment of Spatial Memory in Rats
Abstract
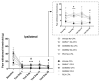
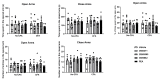
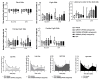
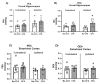
Peroxisome proliferator-activated receptors (PPARs) are ligand-dependent transcription factors that exist in three isoforms: PPARα, PPARβ/δ and PPARγ. Studies suggest that the PPAR signalling system may modulate pain, anxiety and cognition. The aim of the present study was to investigate whether endogenous signalling via PPARs differentially modulates innate anxiety responses and mnemonic function in the presence and absence of inflammatory pain. We examined the effects of intraperitoneal administration of GW6471 (PPARα antagonist), GSK0660 (PPARβ/δ antagonist), GW9662 (PPARγ antagonist), and N-palmitoylethanolamide (PEA) on rat behaviour in the elevated plus maze (EPM), open field (OF), light-dark box (LDB), and novel object recognition (NOR) tests in the presence or absence of chronic inflammatory pain. Complete Freund's Adjuvant (CFA)-injected rats exhibited impaired recognition and spatial mnemonic performance in the NOR test and pharmacological blockade of PPARα further impaired spatial memory in CFA-treated rats. N-oleoylethanolamide (OEA) levels were higher in the dorsal hippocampus in CFA-injected animals compared to their counterparts. The results suggest a modulatory effect of CFA-induced chronic inflammatory pain on cognitive processing, but not on innate anxiety-related responses. Increased OEA-PPARα signalling may act as a compensatory mechanism to preserve spatial memory function following CFA injection.
Keywords: OEA; PEA; anxiety; cognition; complete Freund adjuvant; dorsal hippocampus; peroxisome-proliferator activated receptor; spatial memory.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Pharmacological Blockade of PPAR Isoforms Increases Conditioned Fear Responding in the Presence of Nociceptive Tone.Molecules. 2020 Feb 24;25(4):1007. doi: 10.3390/molecules25041007. Molecules. 2020. PMID: 32102354 Free PMC article.
-
Characterisation of peroxisome proliferator-activated receptor signalling in the midbrain periaqueductal grey of rats genetically prone to heightened stress, negative affect and hyperalgesia.Brain Res. 2017 Feb 15;1657:185-192. doi: 10.1016/j.brainres.2016.11.022. Epub 2016 Dec 1. Brain Res. 2017. PMID: 27916440
-
Effects of Intra-BLA Administration of PPAR Antagonists on Formalin-Evoked Nociceptive Behaviour, Fear-Conditioned Analgesia, and Conditioned Fear in the Presence or Absence of Nociceptive Tone in Rats.Molecules. 2022 Mar 21;27(6):2021. doi: 10.3390/molecules27062021. Molecules. 2022. PMID: 35335382 Free PMC article.
-
Neuroprotective properties of peroxisome proliferator-activated receptor alpha (PPARα) and its lipid ligands.Curr Med Chem. 2014;21(24):2803-21. doi: 10.2174/0929867321666140303143455. Curr Med Chem. 2014. PMID: 24606520 Review.
-
Peroxisome proliferator-activated receptor (PPAR)β/δ, a possible nexus of PPARα- and PPARγ-dependent molecular pathways in neurodegenerative diseases: Review and novel hypotheses.Neurochem Int. 2013 Oct;63(4):322-30. doi: 10.1016/j.neuint.2013.06.012. Epub 2013 Jun 25. Neurochem Int. 2013. PMID: 23811400 Review.
Cited by
-
Stress-induced reduction of Na+/H+ exchanger isoform 1 promotes maladaptation of neuroplasticity and exacerbates depressive behaviors.Sci Adv. 2022 Nov 11;8(45):eadd7063. doi: 10.1126/sciadv.add7063. Epub 2022 Nov 11. Sci Adv. 2022. PMID: 36367929 Free PMC article.
-
Palmitoylethanolamide causes dose-dependent changes in brain function and the lipidome.Front Neurosci. 2024 Nov 27;18:1506352. doi: 10.3389/fnins.2024.1506352. eCollection 2024. Front Neurosci. 2024. PMID: 39664446 Free PMC article.
-
Fatty acid binding protein 5 inhibition attenuates pronociceptive cytokine/chemokine expression and suppresses osteoarthritis pain: A comparative human and rat study.Osteoarthritis Cartilage. 2024 Mar;32(3):266-280. doi: 10.1016/j.joca.2023.11.010. Epub 2023 Nov 28. Osteoarthritis Cartilage. 2024. PMID: 38035977 Free PMC article.
-
Characterization of pain-, anxiety-, and cognition-related behaviors in the complete Freund's adjuvant model of chronic inflammatory pain in Wistar-Kyoto rats.Front Pain Res (Lausanne). 2023 Apr 11;4:1131069. doi: 10.3389/fpain.2023.1131069. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37113211 Free PMC article.
-
Therapeutic effect of palmitoylethanolamide in cognitive decline: A systematic review and preliminary meta-analysis of preclinical and clinical evidence.Front Psychiatry. 2022 Oct 28;13:1038122. doi: 10.3389/fpsyt.2022.1038122. eCollection 2022. Front Psychiatry. 2022. PMID: 36387000 Free PMC article.
References
-
- Rockwell C.E., Snider N.T., Thompson J.T., Heuvel J.P.V., Kaminski N.E. Interleukin-2 Suppression by 2-Arachidonyl Glycerol Is Mediated through Peroxisome Proliferator-Activated Receptor γ Independently of Cannabinoid Receptors 1 and 2. Mol. Pharmacol. 2006;70:101–111. doi: 10.1124/mol.105.019117. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

