Genome-Wide Screening of Oxidizing Agent Resistance Genes in Escherichia coli
- PMID: 34072091
- PMCID: PMC8228696
- DOI: 10.3390/antiox10060861
Genome-Wide Screening of Oxidizing Agent Resistance Genes in Escherichia coli
Abstract
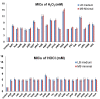
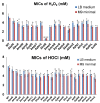
The use of oxidizing agents is one of the most favorable approaches to kill bacteria in daily life. However, bacteria have been evolving to survive in the presence of different oxidizing agents. In this study, we aimed to obtain a comprehensive list of genes whose expression can make Escherichiacoli cells resistant to different oxidizing agents. For this purpose, we utilized the ASKA library and performed a genome-wide screening of ~4200 E. coli genes. Hydrogen peroxide (H2O2) and hypochlorite (HOCl) were tested as representative oxidizing agents in this study. To further validate our screening results, we used different E. coli strains as host cells to express or inactivate selected resistance genes individually. More than 100 genes obtained in this screening were not known to associate with oxidative stress responses before. Thus, this study is expected to facilitate both basic studies on oxidative stress and the development of antibacterial agents.
Keywords: AKSA library; genome-wide screening; hydrogen peroxide; hypochlorite; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

