Comparison of Nonheme Manganese- and Iron-Containing Flavone Synthase Mimics
- PMID: 34072092
- PMCID: PMC8198008
- DOI: 10.3390/molecules26113220
Comparison of Nonheme Manganese- and Iron-Containing Flavone Synthase Mimics
Abstract
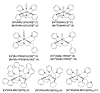
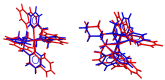
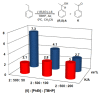
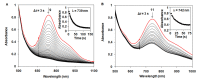
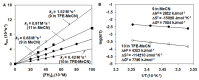
Heme and nonheme-type flavone synthase enzymes, FS I and FS II are responsible for the synthesis of flavones, which play an important role in various biological processes, and have a wide range of biomedicinal properties including antitumor, antimalarial, and antioxidant activities. To get more insight into the mechanism of this curious enzyme reaction, nonheme structural and functional models were carried out by the use of mononuclear iron, [FeII(CDA-BPA*)]2+ (6) [CDA-BPA = N,N,N',N'-tetrakis-(2-pyridylmethyl)-cyclohexanediamine], [FeII(CDA-BQA*)]2+ (5) [CDA-BQA = N,N,N',N'-tetrakis-(2-quinolilmethyl)-cyclohexanediamine], [FeII(Bn-TPEN)(CH3CN)]2+ (3) [Bn-TPEN = N-benzyl-N,N',N'-tris(2-pyridylmethyl)-1,2-diaminoethane], [FeIV(O)(Bn-TPEN)]2+ (9), and manganese, [MnII(N4Py*)(CH3CN)]2+ (2) [N4Py* = N,N-bis(2-pyridylmethyl)-1,2-di(2-pyridyl)ethylamine)], [MnII(Bn-TPEN)(CH3CN)]2+ (4) complexes as catalysts, where the possible reactive intermediates, high-valent FeIV(O) and MnIV(O) are known and well characterised. The results of the catalytic and stoichiometric reactions showed that the ligand framework and the nature of the metal cofactor significantly influenced the reactivity of the catalyst and its intermediate. Comparing the reactions of [FeIV(O)(Bn-TPEN)]2+ (9) and [MnIV(O)(Bn-TPEN)]2+ (10) towards flavanone under the same conditions, a 3.5-fold difference in reaction rate was observed in favor of iron, and this value is three orders of magnitude higher than was observed for the previously published [FeIV(O)(N2Py2Q*)]2+ [N,N-bis(2-quinolylmethyl)-1,2-di(2-pyridyl)ethylamine] species.
Keywords: flavone synthase; iron(IV)-oxo; kinetic studies; manganese(IV)-oxo; oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






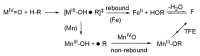
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

