Nebulized Colistin in Ventilator-Associated Pneumonia and Tracheobronchitis: Historical Background, Pharmacokinetics and Perspectives
- PMID: 34072189
- PMCID: PMC8227626
- DOI: 10.3390/microorganisms9061154
Nebulized Colistin in Ventilator-Associated Pneumonia and Tracheobronchitis: Historical Background, Pharmacokinetics and Perspectives
Abstract
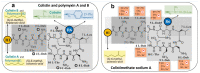
Clinical evidence suggests that nebulized colistimethate sodium (CMS) has benefits for treating lower respiratory tract infections caused by multidrug-resistant Gram-negative bacteria (GNB). Colistin is positively charged, while CMS is negatively charged, and both have a high molecular mass and are hydrophilic. These physico-chemical characteristics impair crossing of the alveolo-capillary membrane but enable the disruption of the bacterial wall of GNB and the aggregation of the circulating lipopolysaccharide. Intravenous CMS is rapidly cleared by glomerular filtration and tubular excretion, and 20-25% is spontaneously hydrolyzed to colistin. Urine colistin is substantially reabsorbed by tubular cells and eliminated by biliary excretion. Colistin is a concentration-dependent antibiotic with post-antibiotic and inoculum effects. As CMS conversion to colistin is slower than its renal clearance, intravenous administration can lead to low plasma and lung colistin concentrations that risk treatment failure. Following nebulization of high doses, colistin (200,000 international units/24h) lung tissue concentrations are > five times minimum inhibitory concentration (MIC) of GNB in regions with multiple foci of bronchopneumonia and in the range of MIC breakpoints in regions with confluent pneumonia. Future research should include: (1) experimental studies using lung microdialysis to assess the PK/PD in the interstitial fluid of the lung following nebulization of high doses of colistin; (2) superiority multicenter randomized controlled trials comparing nebulized and intravenous CMS in patients with pandrug-resistant GNB ventilator-associated pneumonia and ventilator-associated tracheobronchitis; (3) non-inferiority multicenter randomized controlled trials comparing nebulized CMS to intravenous new cephalosporines/ß-lactamase inhibitors in patients with extensive drug-resistant GNB ventilator-associated pneumonia and ventilator-associated tracheobronchitis.
Keywords: colistin; multidrug resistant gram-negative bacteria; nebulized colistimethate sodium; nebulized polymyxin; pharmacodynamics; phramacokinetic; polylyxin resistance; technique of nebulization; ventilator-associated pneumonia; ventilator-associated tracheobronchitis.
Conflict of interest statement
The authors declare receiving consulting fees, unrestricted research grants and equipment research support from Aerogen Ltd., unrestricted research grant, speaker fees, travel reimbursements from Fisher & Paykel, unrestricted research grant form Hamilton medical, consulting fees from La Diffusion Technique Française. M.L. is a consultant for Gilead and Amomed and gave lectures for Aspen and MSD. P.F.L. is a consultant for Adrenomed and Inotrem and received an unrestricted research grant from Aerogen. L.P. declares that Stony Brook University holds patents on targeted antibiotic therapy to intubated patients licensed to InspiRx, Inc. and that she serves as a consultant to InspiRx and is a member of Merck’s Advisory Committee for Gram-negative pneumonias. JRe received grant support from Bayer and served in the advisory board for Bayer and speakers bureau for Norma Helas. A.T. declares participating to the advisory board of Cardeas, Bayer and Polyphor and receiving unrestricted research grants from the three companies. T.W. received grant support from German Research Council, German MInistry of Research and Education, received fees for lectures from AstraZeneca, Basilea, Bayer, GSK, Infectopharm, MSD, Novartis, Pfizer, Roche and declares participating to the advisory board of AstraZeneca, Basilea, Bayer, GSK, Novartis, Pfizer, Roche. The other authors declare no conflict of interest. K.P. received lecture fees from MSD Greece and Pfizer Hellas.
Figures




References
-
- World Health Organization Critically important antimicrobials for human medicine 3rd Revision 2011. [(accessed on 22 May 2021)];Clin. Infect. Dis. 2012 55:712–719. Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-en.... - PubMed
-
- European Medicine Agency Updated Advice on the Use of Colistin Products in Animals within the European Union: Development of Resistance and Possible Impact in Human and Animals Health (EMA/CVMP/CHMP/231573/2016) [(accessed on 22 May 2021)];2016 Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/updated-advi....
-
- Greenfield S., Teres D., Bushnell L.S., Hedley-Whyte J., Feingold D.S. Prevention of gram-negative bacillary pneumonia using aerosol polymyxin as prophylaxis. I. Effect on the colonization pattern of the upper respiratory tract of seriously ill patients. J. Clin. Investig. 1973;52:2935–2940. doi: 10.1172/JCI107490. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials

