Psychological Distress of Metastatic Melanoma Patients during Treatment with Immune Checkpoint Inhibitors: Results of a Prospective Study
- PMID: 34072206
- PMCID: PMC8198252
- DOI: 10.3390/cancers13112642
Psychological Distress of Metastatic Melanoma Patients during Treatment with Immune Checkpoint Inhibitors: Results of a Prospective Study
Abstract
Background: Immune checkpoint inhibitors (ICI) provide effective treatment options for advanced melanoma patients. However, they are associated with high rates of immune-related side effects. There are no data on the distress of melanoma patients during their ICI treatment. We, therefore, conducted a prospective longitudinal study to assess distress and the need for psycho-oncological support in these patients.
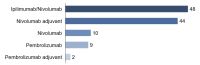
Methods: Questionnaires were completed before initiation of ICI (T0), after 6-8 weeks (T1), and after 12-14 weeks (T2). We furthermore included the Hornheide Screening Instrument (HSI), distress thermometer (DT), and patients' self-assessment. Binary logistic regression was performed to identify factors indicating a need for psychooncological support.
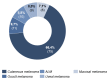
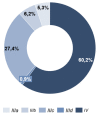
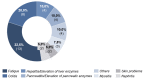
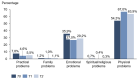
Results: 36.3%/55.8% (HSI / DT) of the patients were above the threshold, indicating a need for psychooncological support at T0, and 7.8% of the patients reported practical problems. In contrast, at T2, the distress values had decreased to 29.0%/40.2% (HSI/DT), respectively. Female gender and occurrence of side effects significantly correlated to values above the threshold. The strongest factor was the patient's self-assessment.
Conclusion: With the beginning of ICI, psychooncological support should be offered. Furthermore, practical problems should be considered, e.g., transport to therapy. Female patients and patients with side effects should be given special attention, as well as the patient self-assessment.
Keywords: checkpoint inhibitors; distress; dt; his; immune therapy; metastatic melanoma; problems; psychooncology.
Conflict of interest statement
A.F. served as a consultant to Roche, Novartis, MSD, BMS, Pierre-Fabre; received travel support from Roche, Novartis, BMS, Pierre-Fabre, received speaker fees from Roche, Novartis, BMS, MSD, and CeGaT, outside the submitted work. She reports institutional research grants from BMS Stiftung Immunonkologie outside the submitted work. T.E. reports personal fees from Amgen, grants and personal fees from Novartis, personal fees from Philogen, grants, and personal fees from Roche, grants and personal fees from Sanofi, personal fees from BMS, personal fees from MSD, outside the submitted work. C.G. reports personal fees from Amgen, grants and personal fees from NeraCare, grants and personal fees from Novartis, personal fees from Philogen, grants and personal fees from Roche, grants and personal fees from Sanofi, personal fees from BMS, personal fees from MSD, outside the submitted work. The other authors state no conflict of interest.
Figures
References
-
- Forschner A., Riedel P., Gassenmaier M., Scheu A., Kofler L., Garbe C., Schäffeler N., Mayer S., Eigentler T.K. The demand for psycho-oncological support in 820 melanoma patients: What are the determinants for the development of distress? JCO. 2017;35(Suppl. 15):9514. doi: 10.1200/JCO.2017.35.15_suppl.9514. - DOI
LinkOut - more resources
Full Text Sources