Light-Addressable Actuator-Sensor Platform for Monitoring and Manipulation of pH Gradients in Microfluidics: A Case Study with the Enzyme Penicillinase
- PMID: 34072213
- PMCID: PMC8230332
- DOI: 10.3390/bios11060171
Light-Addressable Actuator-Sensor Platform for Monitoring and Manipulation of pH Gradients in Microfluidics: A Case Study with the Enzyme Penicillinase
Abstract
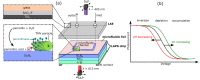
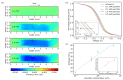
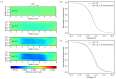
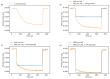
The feasibility of light-addressed detection and manipulation of pH gradients inside an electrochemical microfluidic cell was studied. Local pH changes, induced by a light-addressable electrode (LAE), were detected using a light-addressable potentiometric sensor (LAPS) with different measurement modes representing an actuator-sensor system. Biosensor functionality was examined depending on locally induced pH gradients with the help of the model enzyme penicillinase, which had been immobilized in the microfluidic channel. The surface morphology of the LAE and enzyme-functionalized LAPS was studied by scanning electron microscopy. Furthermore, the penicillin sensitivity of the LAPS inside the microfluidic channel was determined with regard to the analyte's pH influence on the enzymatic reaction rate. In a final experiment, the LAE-controlled pH inhibition of the enzyme activity was monitored by the LAPS.
Keywords: actuator-sensor system; enzyme kinetics; light-addressable electrode; light-addressable potentiometric sensor; microfluidics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Light-Addressable Potentiometric Sensors in Microfluidics.Front Bioeng Biotechnol. 2022 Feb 21;10:833481. doi: 10.3389/fbioe.2022.833481. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35265603 Free PMC article. Review.
-
Actively Driven Light-Addressable Sensor/Actuator System for Automated pH Control for the Integration in Lab-On-A-Chip (LoC) Platforms.ACS Sens. 2024 Mar 22;9(3):1533-1544. doi: 10.1021/acssensors.3c02712. Epub 2024 Mar 6. ACS Sens. 2024. PMID: 38445576
-
Highly sensitive covalently functionalized light-addressable potentiometric sensor for determination of biomarker.Mater Sci Eng C Mater Biol Appl. 2016 Jun;63:185-91. doi: 10.1016/j.msec.2016.02.064. Epub 2016 Feb 26. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27040210
-
Modulated light-activated electrochemistry at silicon functionalized with metal-organic frameworks towards addressable DNA chips.Biosens Bioelectron. 2019 Dec 15;146:111750. doi: 10.1016/j.bios.2019.111750. Epub 2019 Oct 3. Biosens Bioelectron. 2019. PMID: 31605989
-
The light-addressable potentiometric sensor for multi-ion sensing and imaging.Methods. 2005 Sep;37(1):94-102. doi: 10.1016/j.ymeth.2005.05.020. Epub 2005 Sep 30. Methods. 2005. PMID: 16199169 Review.
Cited by
-
Towards Multi-Analyte Detection with Field-Effect Capacitors Modified with Tobacco Mosaic Virus Bioparticles as Enzyme Nanocarriers.Biosensors (Basel). 2022 Jan 14;12(1):43. doi: 10.3390/bios12010043. Biosensors (Basel). 2022. PMID: 35049671 Free PMC article.
-
Facile Purification and Use of Tobamoviral Nanocarriers for Antibody-Mediated Display of a Two-Enzyme System.Viruses. 2023 Sep 19;15(9):1951. doi: 10.3390/v15091951. Viruses. 2023. PMID: 37766357 Free PMC article.
-
Selective Detection of Penicillin G Antibiotic in Milk by Molecularly Imprinted Polymer-Based Plasmonic SPR Sensor.Biomimetics (Basel). 2021 Dec 14;6(4):72. doi: 10.3390/biomimetics6040072. Biomimetics (Basel). 2021. PMID: 34940015 Free PMC article.
-
Light Addressable Potentiometric Sensors for Biochemical Imaging on Microscale: A Review on Optimization of Imaging Speed and Spatial Resolution.ACS Omega. 2023 Nov 3;8(45):42028-42044. doi: 10.1021/acsomega.3c04789. eCollection 2023 Nov 14. ACS Omega. 2023. PMID: 38024735 Free PMC article. Review.
-
Light-Addressable Potentiometric Sensors in Microfluidics.Front Bioeng Biotechnol. 2022 Feb 21;10:833481. doi: 10.3389/fbioe.2022.833481. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35265603 Free PMC article. Review.
References
-
- Mousavi Shaegh S.A., de Ferrari F., Zhang Y.S., Nabavinia M., Mohammad N.B., Ryan J., Pourmand A., Laukaitis E., Banan Sadeghian R., Nadhman A., et al. A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics. 2016;10:044111. doi: 10.1063/1.4955155. - DOI - PMC - PubMed
-
- Meller K., Szumski M., Buszewski B. Microfluidic reactors with immobilized enzymes—Characterization, dividing, perspectives. Sens. Actuators B. 2017;244:84–106. doi: 10.1016/j.snb.2016.12.021. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

