Current Perspectives on the Physiological Activities of Fermented Soybean-Derived Cheonggukjang
- PMID: 34072216
- PMCID: PMC8198423
- DOI: 10.3390/ijms22115746
Current Perspectives on the Physiological Activities of Fermented Soybean-Derived Cheonggukjang
Abstract
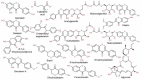
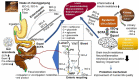
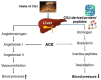
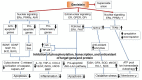
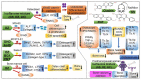
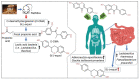
Cheonggukjang (CGJ, fermented soybean paste), a traditional Korean fermented dish, has recently emerged as a functional food that improves blood circulation and intestinal regulation. Considering that excessive consumption of refined salt is associated with increased incidence of gastric cancer, high blood pressure, and stroke in Koreans, consuming CGJ may be desirable, as it can be made without salt, unlike other pastes. Soybeans in CGJ are fermented by Bacillus strains (B. subtilis or B. licheniformis), Lactobacillus spp., Leuconostoc spp., and Enterococcus faecium, which weaken the activity of putrefactive bacteria in the intestines, act as antibacterial agents against pathogens, and facilitate the excretion of harmful substances. Studies on CGJ have either focused on improving product quality or evaluating the bioactive substances contained in CGJ. The fermentation process of CGJ results in the production of enzymes and various physiologically active substances that are not found in raw soybeans, including dietary fiber, phospholipids, isoflavones (e.g., genistein and daidzein), phenolic acids, saponins, trypsin inhibitors, and phytic acids. These components prevent atherosclerosis, oxidative stress-mediated heart disease and inflammation, obesity, diabetes, senile dementia, cancer (e.g., breast and lung), and osteoporosis. They have also been shown to have thrombolytic, blood pressure-lowering, lipid-lowering, antimutagenic, immunostimulatory, anti-allergic, antibacterial, anti-atopic dermatitis, anti-androgenetic alopecia, and anti-asthmatic activities, as well as skin improvement properties. In this review, we examined the physiological activities of CGJ and confirmed its potential as a functional food.
Keywords: bioactive molecule; biological activity; cheonggukjang; fermented soybean paste; human health benefit.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Lee B.Y., Kim D.N., Kim K.H. Physico-chemical properties of viscous substance extracted from chungkook-jang. Korean J. Food Sci. Technol. 1991;23:599–604.
-
- Lee J.O., Ha S.D., Kim A.J., Yuh C.S., Bang I.S., Park S.H. Industrial application and physiological functions of chongkukjang. Food Sci. Ind. 2005;38:69–78.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

