Blood Plasma Quality Control by Plasma Glutathione Status
- PMID: 34072235
- PMCID: PMC8226592
- DOI: 10.3390/antiox10060864
Blood Plasma Quality Control by Plasma Glutathione Status
Abstract
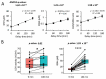
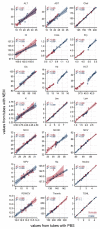
Timely centrifugation of blood for plasma preparation is a key step to ensure high plasma quality for analytics. Delays during preparation can significantly influence readouts of key clinical parameters. However, in a routine clinical environment, a strictly controlled timeline is often not feasible. The next best approach is to control for sample preparation delays by a marker that provides a readout of the time-dependent degradation of the sample. In this study, we explored the usefulness of glutathione status as potential marker of plasma preparation delay. As the concentration of glutathione in erythrocytes is at least two orders of magnitude higher than in plasma, even the slightest leakage of glutathione from the cells can be readily observed. Over the 3 h observation period employed in this study, we observed a linear increase of plasma concentrations of both reduced (GSH) and oxidized glutathione (GSSG). Artificial oxidation of GSH is prevented by rapid alkylation with N-ethylmaleimide directly in the blood sampling vessel as recently published. The observed relative leakage of GSH was significantly higher than that of GSSG. A direct comparison with plasma lactate dehydrogenase activity, a widely employed hemolysis marker, clearly demonstrated the superiority of our approach for quality control. Moreover, we show that the addition of the thiol alkylating reagent NEM directly to the blood tubes does not influence downstream analysis of other clinical parameters. In conclusion, we report that GSH gives an excellent readout of the duration of plasma preparation and the associated pre-analytical errors.
Keywords: blood plasma; clinical chemistry; glutathione; pre-analytical error; quality control; redox status.
Conflict of interest statement
The authors declare no conflict of interest. The authors N.B., A.A.-B. and E.Z. were employed at companies as stated in the affiliations.
Figures

Similar articles
-
Addressing Glutathione Redox Status in Clinical Samples by Two-Step Alkylation with N-ethylmaleimide Isotopologues.Metabolites. 2020 Feb 16;10(2):71. doi: 10.3390/metabo10020071. Metabolites. 2020. PMID: 32079090 Free PMC article.
-
Analysis of GSH and GSSG after derivatization with N-ethylmaleimide.Nat Protoc. 2013 Sep;8(9):1660-9. doi: 10.1038/nprot.2013.095. Epub 2013 Aug 1. Nat Protoc. 2013. PMID: 23928499
-
Validation of a liquid chromatography tandem mass spectrometry method to measure oxidized and reduced forms of glutathione in whole blood and verification in a mouse model as an indicator of oxidative stress.J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Apr 15;1019:45-50. doi: 10.1016/j.jchromb.2015.10.041. Epub 2015 Nov 1. J Chromatogr B Analyt Technol Biomed Life Sci. 2016. PMID: 26575459
-
Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells.Free Radic Biol Med. 2017 Nov;112:360-375. doi: 10.1016/j.freeradbiomed.2017.08.008. Epub 2017 Aug 12. Free Radic Biol Med. 2017. PMID: 28807817 Review.
-
The fairytale of the GSSG/GSH redox potential.Biochim Biophys Acta. 2013 May;1830(5):3139-42. doi: 10.1016/j.bbagen.2012.10.020. Epub 2012 Nov 2. Biochim Biophys Acta. 2013. PMID: 23127894 Review.
Cited by
-
Direct Derivatization in Dried Blood Spots for Oxidized and Reduced Glutathione Quantification in Newborns.Antioxidants (Basel). 2022 Jun 14;11(6):1165. doi: 10.3390/antiox11061165. Antioxidants (Basel). 2022. PMID: 35740062 Free PMC article.
-
Daily Vinegar Ingestion Improves Depression and Enhances Niacin Metabolism in Overweight Adults: A Randomized Controlled Trial.Nutrients. 2024 Jul 18;16(14):2305. doi: 10.3390/nu16142305. Nutrients. 2024. PMID: 39064748 Free PMC article. Clinical Trial.
-
The Effect of Protein Supplementation and Playing Time on Recovery Kinetics During a Congested Basketball Schedule.Nutrients. 2024 Dec 31;17(1):128. doi: 10.3390/nu17010128. Nutrients. 2024. PMID: 39796561 Free PMC article. Clinical Trial.
-
Making bloodwork work: the impact of sample collection, processing, and storage on plasma glutathione measurement, and implications for translation.Transl Psychiatry. 2024 Sep 23;14(1):385. doi: 10.1038/s41398-024-03086-5. Transl Psychiatry. 2024. PMID: 39313523 Free PMC article.
References
-
- Tuck M.K., Chan D.W., Chia D., Godwin A.K., Grizzle W.E., Krueger K.E., Rom W., Sanda M., Sorbara L., Stass S., et al. Standard Operating Procedures for Serum and Plasma Collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group. J. Proteome Res. 2009;8:113–117. doi: 10.1021/pr800545q. - DOI - PMC - PubMed
-
- Ruiz-Godoy L.M., Enríquez-Cárcamo V., Suárez-Roa L., Lopez-Castro M.L., Santamaría A., Orozco-Morales M., Colín-González A.L. Identification of Specific Pre-analytical Quality Control Markers in Plasma and Serum Samples. Anal. Methods. 2019;11:2259–2271. doi: 10.1039/C9AY00131J. - DOI
-
- Kamlage B., Maldonado S.G., Bethan B., Peter E., Schmitz O., Liebenberg V., Schatz P. Quality Markers Addressing Preanalytical Variations of Blood and Plasma Processing Identified by Broad and Targeted Metabolite Profiling. Clin. Chem. 2014;60:399–412. doi: 10.1373/clinchem.2013.211979. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

