Blood Plasma Quality Control by Plasma Glutathione Status
- PMID: 34072235
- PMCID: PMC8226592
- DOI: 10.3390/antiox10060864
Blood Plasma Quality Control by Plasma Glutathione Status
Abstract
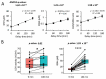
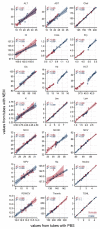
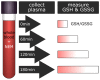
Timely centrifugation of blood for plasma preparation is a key step to ensure high plasma quality for analytics. Delays during preparation can significantly influence readouts of key clinical parameters. However, in a routine clinical environment, a strictly controlled timeline is often not feasible. The next best approach is to control for sample preparation delays by a marker that provides a readout of the time-dependent degradation of the sample. In this study, we explored the usefulness of glutathione status as potential marker of plasma preparation delay. As the concentration of glutathione in erythrocytes is at least two orders of magnitude higher than in plasma, even the slightest leakage of glutathione from the cells can be readily observed. Over the 3 h observation period employed in this study, we observed a linear increase of plasma concentrations of both reduced (GSH) and oxidized glutathione (GSSG). Artificial oxidation of GSH is prevented by rapid alkylation with N-ethylmaleimide directly in the blood sampling vessel as recently published. The observed relative leakage of GSH was significantly higher than that of GSSG. A direct comparison with plasma lactate dehydrogenase activity, a widely employed hemolysis marker, clearly demonstrated the superiority of our approach for quality control. Moreover, we show that the addition of the thiol alkylating reagent NEM directly to the blood tubes does not influence downstream analysis of other clinical parameters. In conclusion, we report that GSH gives an excellent readout of the duration of plasma preparation and the associated pre-analytical errors.
Keywords: blood plasma; clinical chemistry; glutathione; pre-analytical error; quality control; redox status.
Conflict of interest statement
The authors declare no conflict of interest. The authors N.B., A.A.-B. and E.Z. were employed at companies as stated in the affiliations.
Figures

References
-
- Tuck M.K., Chan D.W., Chia D., Godwin A.K., Grizzle W.E., Krueger K.E., Rom W., Sanda M., Sorbara L., Stass S., et al. Standard Operating Procedures for Serum and Plasma Collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group. J. Proteome Res. 2009;8:113–117. doi: 10.1021/pr800545q. - DOI - PMC - PubMed
-
- Ruiz-Godoy L.M., Enríquez-Cárcamo V., Suárez-Roa L., Lopez-Castro M.L., Santamaría A., Orozco-Morales M., Colín-González A.L. Identification of Specific Pre-analytical Quality Control Markers in Plasma and Serum Samples. Anal. Methods. 2019;11:2259–2271. doi: 10.1039/C9AY00131J. - DOI
-
- Kamlage B., Maldonado S.G., Bethan B., Peter E., Schmitz O., Liebenberg V., Schatz P. Quality Markers Addressing Preanalytical Variations of Blood and Plasma Processing Identified by Broad and Targeted Metabolite Profiling. Clin. Chem. 2014;60:399–412. doi: 10.1373/clinchem.2013.211979. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

