Red Blood Cells: Tethering, Vesiculation, and Disease in Micro-Vascular Flow
- PMID: 34072241
- PMCID: PMC8228733
- DOI: 10.3390/diagnostics11060971
Red Blood Cells: Tethering, Vesiculation, and Disease in Micro-Vascular Flow
Abstract
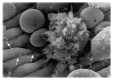
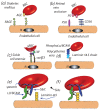
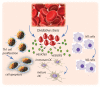
The red blood cell has become implicated in the progression of a range of diseases; mechanisms by which red cells are involved appear to include the transport of inflammatory species via red cell-derived vesicles. We review this role of RBCs in diseases such as diabetes mellitus, sickle cell anemia, polycythemia vera, central retinal vein occlusion, Gaucher disease, atherosclerosis, and myeloproliferative neoplasms. We propose a possibly unifying, and novel, paradigm for the inducement of RBC vesiculation during vascular flow of red cells adhered to the vascular endothelium as well as to the red pulp of the spleen. Indeed, we review the evidence for this hypothesis that links physiological conditions favoring both vesiculation and enhanced RBC adhesion and demonstrate the veracity of this hypothesis by way of a specific example occurring in splenic flow which we argue has various renderings in a wide range of vascular flows, in particular microvascular flows. We provide a mechanistic basis for membrane loss and the formation of lysed red blood cells in the spleen that may mediate their turnover. Our detailed explanation for this example also makes clear what features of red cell deformability are involved in the vesiculation process and hence require quantification and a new form of quantitative indexing.
Keywords: adhesion; hemolysis; vesiculation.
Conflict of interest statement
The authors declare no conflict of interest. There was no role of any funders in the writing or decision to publish this manuscript.
Figures