Hydrogen Peroxide Increases during Endodormancy and Decreases during Budbreak in Grapevine (Vitis vinifera L.) Buds
- PMID: 34072287
- PMCID: PMC8228137
- DOI: 10.3390/antiox10060873
Hydrogen Peroxide Increases during Endodormancy and Decreases during Budbreak in Grapevine (Vitis vinifera L.) Buds
Abstract
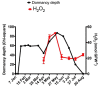
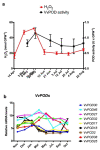
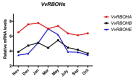
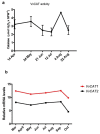
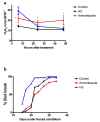
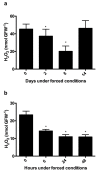
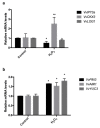
Changes in the level of hydrogen peroxide (H2O2) is a good indicator to monitor fluctuations in cellular metabolism and in the stress responses. In this study, the changes in H2O2 content during bud endodormancy (ED) and budbreak were analysed in grapevine (Vitis vinifera L.). The results showed a gradual increase in the H2O2 content during the development of bud ED, which was mainly due to an increase in the activity of peroxidases (PODs). The maximum H2O2 content reached in the grapevine buds coincided with the maximum depth of bud ED. In contrast, during budbreak, the H2O2 content decreased. As the plant hormones cytokinin (CK) and auxin play an important role in budbreak and growth resumption in grapevine, the effect of exogenous applications of H2O2 on the expression of genes involved in CK and auxin metabolism was analysed. The results showed that H2O2 represses the expression of the CK biosynthesis genes VvIPT3a and VvLOG1 and induces the expression of the CK-inactivating gene VvCKX3, thus reducing potentially the CK content in the grapevine bud. On the other hand, H2O2 induced the expression of the auxin biosynthesis genes VvAMI1 and VvYUC3 and of the auxin transporter gene VvPIN3, thus increasing potentially the auxin content and auxin transport in grapevine buds. In general, the results suggest that H2O2 in grapevine buds is associated with the depth of ED and negatively regulates its budbreak.
Keywords: auxin; budbreak; cytokinin; dormancy; grapevine buds; hydrogen peroxide; peroxidases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Grant T.N.L., Gargrave J., Dami I.E. Morphological physiological, and biochemical changes in Vitis genotype in responses to photoperiod regimes. Am. J. Enol. Vitic. 2013;64:466–475. doi: 10.5344/ajev.2013.13060. - DOI
-
- Cragin J., Serpe M., Keller M., Shellie K. Dormancy and cold hardiness transition in wine grape cultivars Chardonnay and Cabernet Sauvignon. Am. J. Enol. Vitic. 2017;68:195–202. doi: 10.5344/ajev.2016.16078. - DOI
-
- Lang G.A. Dormancy: A new universal terminology. HortScience. 1987;22:817–820.
-
- Or E., Belausov E., Popilevski I., Tal Y.B. Changes in endogenous ABA level in relation to the dormancy cycle in grapevines grown in a hot climate. J. Hortic. Sci. Biotechnol. 2000;75:190–194. doi: 10.1080/14620316.2000.11511221. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

