Formulation of Piperine-Chitosan-Coated Liposomes: Characterization and In Vitro Cytotoxic Evaluation
- PMID: 34072306
- PMCID: PMC8198173
- DOI: 10.3390/molecules26113281
Formulation of Piperine-Chitosan-Coated Liposomes: Characterization and In Vitro Cytotoxic Evaluation
Abstract
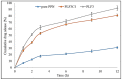
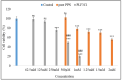
The present research work is designed to prepare and evaluate piperine liposomes and piperine-chitosan-coated liposomes for oral delivery. Piperine (PPN) is a water-insoluble bioactive compound used for different diseases. The prepared formulations were evaluated for physicochemical study, mucoadhesive study, permeation study and in vitro cytotoxic study using the MCF7 breast cancer cell line. Piperine-loaded liposomes (PLF) were prepared by the thin-film evaporation method. The selected liposomes were coated with chitosan (PLFC) by electrostatic deposition to enhance the mucoadhesive property and in vitro therapeutic efficacy. Based on the findings of the study, the prepared PPN liposomes (PLF3) and chitosan coated PPN liposomes (PLF3C1) showed a nanometric size range of 165.7 ± 7.4 to 243.4 ± 7.5, a narrow polydispersity index (>0.3) and zeta potential (-7.1 to 29.8 mV). The average encapsulation efficiency was found to be between 60 and 80% for all prepared formulations. The drug release and permeation study profile showed biphasic release behavior and enhanced PPN permeation. The in vitro antioxidant study results showed a comparable antioxidant activity with pure PPN. The anticancer study depicted that the cell viability assay of tested PLF3C2 has significantly (p < 0.001)) reduced the IC50 when compared with pure PPN. The study revealed that oral chitosan-coated liposomes are a promising delivery system for the PPN and can increase the therapeutic efficacy against the breast cancer cell line.
Keywords: MCF-7; breast cancer; chitosan; liposomes; mucoadhesive; piperine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Alshehri S., Imam S.S., Hussain M.A. Altamimi Formulation of Piperine Ternary Inclusion Complex Using β CD and HPMC: Physicochemical Characterization, Molecular Docking, and Antimicrobial Testing. Processes. 2020;8:1450. doi: 10.3390/pr8111450. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

