Experimental Assessment of Possible Factors Associated with Tick-Borne Encephalitis Vaccine Failure
- PMID: 34072340
- PMCID: PMC8229799
- DOI: 10.3390/microorganisms9061172
Experimental Assessment of Possible Factors Associated with Tick-Borne Encephalitis Vaccine Failure
Abstract
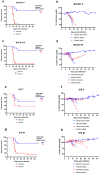
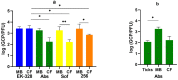
Currently the only effective measure against tick-borne encephalitis (TBE) is vaccination. Despite the high efficacy of approved vaccines against TBE, rare cases of vaccine failures are well documented. Both host- and virus-related factors can account for such failures. In this work, we studied the influence of mouse strain and sex and the effects of cyclophosphamide-induced immunosuppression on the efficacy of an inactivated TBE vaccine. We also investigated how an increased proportion of non-infectious particles in the challenge TBE virus would affect the protectivity of the vaccine. The vaccine efficacy was assessed by mortality, morbidity, levels of viral RNA in the brain of surviving mice, and neutralizing antibody (NAb) titers against the vaccine strain and the challenge virus. Two-dose vaccination protected most animals against TBE symptoms and death, and protectivity depended on strain and sex of mice. Immunosuppression decreased the vaccine efficacy in a dose-dependent manner and changed the vaccine-induced NAb spectrum. The vaccination protected mice against TBE virus neuroinvasion and persistence. However, viral RNA was detected in the brain of some asymptomatic animals at 21 and 42 dpi. Challenge with TBE virus enriched with non-infectious particles led to lower NAb titers in vaccinated mice after the challenge but did not affect the protective efficacy.
Keywords: TBEV; cyclophosphamide; flavivirus; immunosuppression; mouse model; neuroinvasion; non-infectious virus particles; structural heterogeneity; tick-borne encephalitis; vaccine failure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ruzek D., Županc T.A., Borde J., Chrdle A., Eyer L., Karganova G., Kholodilov I., Knap N., Kozlovskaya L., Matveev A., et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antivir. Res. 2019;164:23–51. doi: 10.1016/j.antiviral.2019.01.014. - DOI - PubMed
-
- Domnich A., Panatto D., Arbuzova E.K., Signori A., Avio U., Gasparini R., Amicizia D. Immunogenicity against Far Eastern and Siberian subtypes of tick-borne encephalitis (TBE) virus elicited by the currently available vaccines based on the European subtype: Systematic review and meta-analysis. Hum. Vaccines Immunother. 2014;10:2819–2833. doi: 10.4161/hv.29984. - DOI - PMC - PubMed
-
- Hansson K.E., Rosdahl A., Insulander M., Vene S., Lindquist L., Gredmark-Russ S., Askling H.H. Tick-borne Encephalitis Vaccine Failures: A 10-year Retrospective Study Supporting the Rationale for Adding an Extra Priming Dose in Individuals Starting at Age 50 Years. Clin. Infect. Dis. 2020;70:245–251. doi: 10.1093/cid/ciz176. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

