Enzyme-Responsive Nanoparticles and Coatings Made from Alginate/Peptide Ciprofloxacin Conjugates as Drug Release System
- PMID: 34072352
- PMCID: PMC8226786
- DOI: 10.3390/antibiotics10060653
Enzyme-Responsive Nanoparticles and Coatings Made from Alginate/Peptide Ciprofloxacin Conjugates as Drug Release System
Abstract
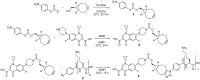
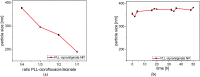
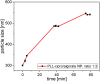
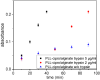
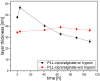
Infection-controlled release of antibacterial agents is of great importance, particularly for the control of peri-implant infections in the postoperative phase. Polymers containing antibiotics bound via enzymatically cleavable linkers could provide access to drug release systems that could accomplish this. Dispersions of nanogels were prepared by ionotropic gelation of alginate with poly-l-lysine, which was conjugated with ciprofloxacin as model drug via a copper-free 1,3-dipolar cycloaddition (click reaction). The nanogels are stable in dispersion and form films which are stable in aqueous environments. However, both the nanogels and the layers are degraded in the presence of an enzyme and the ciprofloxacin is released. The efficacy of the released drug against Staphylococcus aureus is negatively affected by the residues of the linker. Both the acyl modification of the amine nitrogen in ciprofloxacin and the sterically very demanding linker group with three annellated rings could be responsible for this. However the basic feasibility of the principle for enzyme-triggered release of drugs was successfully demonstrated.
Keywords: ciprofloxacin; copper-free click chemistry; drug release system; enzyme triggered release; implant coating; nanoparticle; peri-implantitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Jiskoot W., Randolph T.W., Volkin D.B., Middaugh C.R., Schöneich C., Winter G., Friess W., Crommelin D.J.A., Carpenter J.F. Protein instability and immunogenicity: Roadblocks to clinical application of injectable protein delivery systems for sustained release. J. Pharm. Sci. 2012;101:946–954. doi: 10.1002/jps.23018. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

