Activation of Anopheles stephensi Pantothenate Kinase and Coenzyme A Biosynthesis Reduces Infection with Diverse Plasmodium Species in the Mosquito Host
- PMID: 34072373
- PMCID: PMC8228300
- DOI: 10.3390/biom11060807
Activation of Anopheles stephensi Pantothenate Kinase and Coenzyme A Biosynthesis Reduces Infection with Diverse Plasmodium Species in the Mosquito Host
Abstract
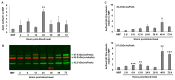
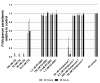
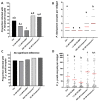
Malaria parasites require pantothenate from both human and mosquito hosts to synthesize coenzyme A (CoA). Specifically, mosquito-stage parasites cannot synthesize pantothenate de novo or take up preformed CoA from the mosquito host, making it essential for the parasite to obtain pantothenate from mosquito stores. This makes pantothenate utilization an attractive target for controlling sexual stage malaria parasites in the mosquito. CoA is synthesized from pantothenate in a multi-step pathway initiated by the enzyme pantothenate kinase (PanK). In this work, we manipulated A. stephensi PanK activity and assessed the impact of mosquito PanK activity on the development of two malaria parasite species with distinct genetics and life cycles: the human parasite Plasmodium falciparum and the mouse parasite Plasmodium yoelii yoelii 17XNL. We identified two putative A. stephensi PanK isoforms encoded by a single gene and expressed in the mosquito midgut. Using both RNAi and small molecules with reported activity against human PanK, we confirmed that A. stephensi PanK manipulation was associated with corresponding changes in midgut CoA levels. Based on these findings, we used two small molecule modulators of human PanK activity (PZ-2891, compound 7) at reported and ten-fold EC50 doses to examine the effects of manipulating A. stephensi PanK on malaria parasite infection success. Our data showed that oral provisioning of 1.3 nM and 13 nM PZ-2891 increased midgut CoA levels and significantly decreased infection success for both Plasmodium species. In contrast, oral provisioning of 62 nM and 620 nM compound 7 decreased CoA levels and significantly increased infection success for both Plasmodium species. This work establishes the A. stephensi CoA biosynthesis pathway as a potential target for broadly blocking malaria parasite development in anopheline hosts. We envision this strategy, with small molecule PanK modulators delivered to mosquitoes via attractive bait stations, working in concert with deployment of parasite-directed novel pantothenamide drugs to block parasite infection in the human host. In mosquitoes, depletion of pantothenate through manipulation to increase CoA biosynthesis is expected to negatively impact Plasmodium survival by starving the parasite of this essential nutrient. This has the potential to kill both wild type parasites and pantothenamide-resistant parasites that could develop under pantothenamide drug pressure if these compounds are used as future therapeutics for human malaria.
Keywords: Anopheles stephensi; CoA; PZ-2891; PanK; Plasmodium falciparum; Plasmodium yoelii; coenzyme A; compound 7; malaria; midgut; pantothenate kinase; small molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sinka M., Pironon S., Massey N., Longbottom J., Hemingway J., Moyes C., Willis K. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. USA. 2020;117:24900–24908. doi: 10.1073/pnas.2003976117. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

