Protective Effects of α-Lipoic Acid and Chlorogenic Acid on Cadmium-Induced Liver Injury in Three-Yellow Chickens
- PMID: 34072384
- PMCID: PMC8228482
- DOI: 10.3390/ani11061606
Protective Effects of α-Lipoic Acid and Chlorogenic Acid on Cadmium-Induced Liver Injury in Three-Yellow Chickens
Abstract
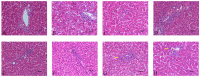
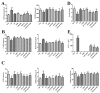
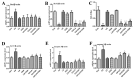
Cadmium (Cd) is a type of noxious heavy metal that is distributed widely. It can severely injure the hepatocytes and cause liver dysfunction by inducing oxidative stress and mitochondrial damage. We evaluated the protective effects of α-lipoic acid (α-LA) or chlorogenic acid (CGA) and their combination on counteracting cadmium toxicity in vivo in three-yellow chickens. For three months, CdCl2 (50 mg/L) was administrated through their drinking water, α-LA (400 mg/kg) was added to feed and CGA (45 mg/kg) was employed by gavage. The administration of Cd led to variations in growth performance, biochemical markers (of the liver, kidney and heart), hematological parameters, liver histopathology (which suggested hepatic injury) and ultrastructure of hepatocytes. Some antioxidant enzymes and oxidative stress parameters showed significant differences in the Cd-exposure group when compared with the control group. The groups treated with Cd and administrated α-LA or CGA showed significant amelioration with inhibited mitochondrial pathway-induced apoptosis. Combining both drugs was the most effective in reducing Cd toxicity in the liver. In summary, the results demonstrated that α-LA and CGA may be beneficial in alleviating oxidative stress induced by oxygen free radicals and tissue injury resulting from Cd-triggered hepatotoxicity.
Keywords: cadmium; chicken liver damage; chlorogenic acid; oxidative stress; α-lipoic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Treatment of cadmium-induced renal oxidative damage in rats by administration of alpha-lipoic acid.Environ Sci Pollut Res Int. 2017 Jan;24(2):1832-1844. doi: 10.1007/s11356-016-7953-x. Epub 2016 Oct 30. Environ Sci Pollut Res Int. 2017. PMID: 27796992
-
Protection Mechanisms Underlying Oral Administration of Chlorogenic Acid against Cadmium-Induced Hepatorenal Injury Related to Regulating Intestinal Flora Balance.J Agric Food Chem. 2021 Feb 10;69(5):1675-1683. doi: 10.1021/acs.jafc.0c06698. Epub 2021 Jan 26. J Agric Food Chem. 2021. PMID: 33494608
-
Alpha-lipoic acid protects against cadmium-induced neuronal injury by inhibiting the endoplasmic reticulum stress eIF2α-ATF4 pathway in rat cortical neurons in vitro and in vivo.Toxicology. 2019 Feb 15;414:1-13. doi: 10.1016/j.tox.2018.12.005. Epub 2018 Dec 31. Toxicology. 2019. PMID: 30605698
-
Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats.Environ Sci Pollut Res Int. 2019 Jul;26(20):20631-20653. doi: 10.1007/s11356-019-05420-7. Epub 2019 May 18. Environ Sci Pollut Res Int. 2019. PMID: 31104231
-
Chlorogenic acid attenuates cadmium-induced intestinal injury in Sprague-Dawley rats.Food Chem Toxicol. 2019 Nov;133:110751. doi: 10.1016/j.fct.2019.110751. Epub 2019 Aug 4. Food Chem Toxicol. 2019. PMID: 31390532
Cited by
-
Research progress of chlorogenic acid in improving inflammatory diseases.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 Oct 28;48(10):1611-1620. doi: 10.11817/j.issn.1672-7347.2023.230146. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 38432890 Free PMC article. Chinese, English.
-
Chlorogenic acid attenuates liver apoptosis and inflammation in endoplasmic reticulum stress-induced mice.Iran J Basic Med Sci. 2023 Apr;26(4):478-485. doi: 10.22038/IJBMS.2023.66827.14659. Iran J Basic Med Sci. 2023. PMID: 37009010 Free PMC article.
-
Effects of lipoic acid on production performance, meat quality, serum biochemistry and antioxidant function in heat-stressed broilers.Trop Anim Health Prod. 2025 Jan 27;57(2):35. doi: 10.1007/s11250-025-04280-3. Trop Anim Health Prod. 2025. PMID: 39869240
-
Combined Effects of Cadmium and Lead on Growth Performance and Kidney Function in Broiler Chicken.Biol Trace Elem Res. 2025 Jan;203(1):358-373. doi: 10.1007/s12011-024-04173-w. Epub 2024 Apr 8. Biol Trace Elem Res. 2025. PMID: 38589681
-
Tobacco as bioenergy and medical plant for biofuels and bioproduction.Heliyon. 2024 Jul 1;10(13):e33920. doi: 10.1016/j.heliyon.2024.e33920. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39055830 Free PMC article. Review.
References
-
- Cai J., Zhang Y., Yang J., Liu Q., Zhao R., Hamid S., Wang H., Xu S., Zhang Z. Antagonistic effects of selenium against necroptosis injury via adiponectin-necrotic pathway induced by cadmium in heart of chicken. RSC Adv. 2017;7:44438–44446. doi: 10.1039/C7RA07952D. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

