Inhibition of Phosphatidylinositol 3-Kinase by Pictilisib Blocks Influenza Virus Propagation in Cells and in Lungs of Infected Mice
- PMID: 34072389
- PMCID: PMC8228449
- DOI: 10.3390/biom11060808
Inhibition of Phosphatidylinositol 3-Kinase by Pictilisib Blocks Influenza Virus Propagation in Cells and in Lungs of Infected Mice
Abstract
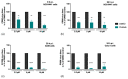
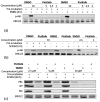
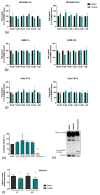
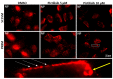
Influenza virus (IV) infections are considered to cause severe diseases of the respiratory tract. Beyond mild symptoms, the infection can lead to respiratory distress syndrome and multiple organ failure. Occurrence of resistant seasonal and pandemic strains against the currently licensed antiviral medications points to the urgent need for new and amply available anti-influenza drugs. Interestingly, the virus-supportive function of the cellular phosphatidylinositol 3-kinase (PI3K) suggests that this signaling module may be a potential target for antiviral intervention. In the sense of repurposing existing drugs for new indications, we used Pictilisib, a known PI3K inhibitor to investigate its effect on IV infection, in mono-cell-culture studies as well as in a human chip model. Our results indicate that Pictilisib is a potent inhibitor of IV propagation already at early stages of infection. In a murine model of IV pneumonia, the in vitro key findings were verified, showing reduced viral titers as well as inflammatory response in the lung after delivery of Pictilisib. Our data identified Pictilisib as a promising drug candidate for anti-IV therapies that warrant further studying. These results further led to the conclusion that the repurposing of previously approved substances represents a cost-effective and efficient way for development of novel antiviral strategies.
Keywords: Pictilisib; influenza virus; phosphatidylinositol-3 kinase; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Liu Shen Wan inhibits influenza a virus and excessive virus-induced inflammatory response via suppression of TLR4/NF-κB signaling pathway in vitro and in vivo.J Ethnopharmacol. 2020 Apr 24;252:112584. doi: 10.1016/j.jep.2020.112584. Epub 2020 Jan 21. J Ethnopharmacol. 2020. PMID: 31972325
-
Unexpected Functional Divergence of Bat Influenza Virus NS1 Proteins.J Virol. 2018 Feb 12;92(5):e02097-17. doi: 10.1128/JVI.02097-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237829 Free PMC article.
-
Inhibition of the infectivity and inflammatory response of influenza virus by Arbidol hydrochloride in vitro and in vivo (mice and ferret).Biomed Pharmacother. 2017 Jul;91:393-401. doi: 10.1016/j.biopha.2017.04.091. Epub 2017 May 2. Biomed Pharmacother. 2017. PMID: 28475918
-
Repurposing Papaverine as an Antiviral Agent against Influenza Viruses and Paramyxoviruses.J Virol. 2020 Feb 28;94(6):e01888-19. doi: 10.1128/JVI.01888-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31896588 Free PMC article.
-
Antiviral efficacy against influenza virus and pharmacokinetic analysis of a novel MEK-inhibitor, ATR-002, in cell culture and in the mouse model.Antiviral Res. 2020 Jun;178:104806. doi: 10.1016/j.antiviral.2020.104806. Epub 2020 Apr 15. Antiviral Res. 2020. PMID: 32304723
Cited by
-
Pathogenicity and virulence of influenza.Virulence. 2023 Dec;14(1):2223057. doi: 10.1080/21505594.2023.2223057. Virulence. 2023. PMID: 37339323 Free PMC article. Review.
-
Age-related STING suppression in macrophages contributes to increased viral load during influenza a virus infection.Immun Ageing. 2024 Nov 14;21(1):80. doi: 10.1186/s12979-024-00482-9. Immun Ageing. 2024. PMID: 39543713 Free PMC article.
-
Women in the European Virus Bioinformatics Center.Viruses. 2022 Jul 12;14(7):1522. doi: 10.3390/v14071522. Viruses. 2022. PMID: 35891501 Free PMC article.
-
Influenza Virus-Induced Paracrine Cellular Senescence of the Lung Contributes to Enhanced Viral Load.Aging Dis. 2023 Aug 1;14(4):1331-1348. doi: 10.14336/AD.2023.0310. Aging Dis. 2023. PMID: 37163429 Free PMC article.
-
Metabolic Modifications by Common Respiratory Viruses and Their Potential as New Antiviral Targets.Viruses. 2021 Oct 14;13(10):2068. doi: 10.3390/v13102068. Viruses. 2021. PMID: 34696497 Free PMC article. Review.
References
-
- Chow E.J., Rolfes M.A., O’Halloran A., Alden N.B., Anderson E.J., Bennett N.M., Billing L., Dufort E., Kirley P.D., George A., et al. Respiratory and Nonrespiratory Diagnoses Associated with Influenza in Hospitalized Adults. JAMA Netw. Open. 2020;3:e201323. doi: 10.1001/jamanetworkopen.2020.1323. - DOI - PMC - PubMed
-
- Jorgensen P., Mereckiene J., Cotter S., Johansen K., Tsolova S., Brown C. How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine. 2018;36:442–452. doi: 10.1016/j.vaccine.2017.12.019. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

