Insights into SARS-CoV-2 Persistence and Its Relevance
- PMID: 34072390
- PMCID: PMC8228265
- DOI: 10.3390/v13061025
Insights into SARS-CoV-2 Persistence and Its Relevance
Abstract
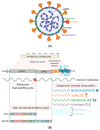
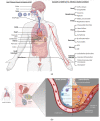
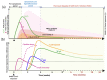
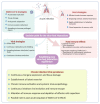
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), continues to wreak havoc, threatening the public health services and imposing economic collapse worldwide. Tailoring public health responses to the SARS-CoV-2 pandemic depends on understanding the mechanism of viral replication, disease pathogenesis, accurately identifying acute infections, and mapping the spreading risk of hotspots across the globe. However, effective identification and isolation of persons with asymptomatic and mild SARS-CoV-2 infections remain the major obstacles to efforts in controlling the SARS-CoV-2 spread and hence the pandemic. Understanding the mechanism of persistent viral shedding, reinfection, and the post-acute sequalae of SARS-CoV-2 infection (PASC) is crucial in our efforts to combat the pandemic and provide better care and rehabilitation to survivors. Here, we present a living literature review (January 2020 through 15 March 2021) on SARS-CoV-2 viral persistence, reinfection, and PASC. We also highlight potential areas of research to uncover putative links between viral persistence, intra-host evolution, host immune status, and protective immunity to guide and direct future basic science and clinical research priorities.
Keywords: COVID-19; PASC; SARS-CoV-2; coronaviruses; long COVID; reinfection; viral persistence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO Coronavirus Disease (COVID-19) Pandemic. [(accessed on 19 April 2021)]; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

