Metabolic Alterations in Sepsis
- PMID: 34072402
- PMCID: PMC8197843
- DOI: 10.3390/jcm10112412
Metabolic Alterations in Sepsis
Abstract
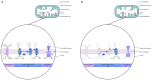
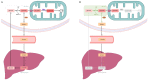
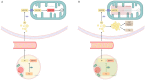
Sepsis is defined as "life-threatening organ dysfunction caused by a dysregulated host response to infection". Contrary to the older definitions, the current one not only focuses on inflammation, but points to systemic disturbances in homeostasis, including metabolism. Sepsis leads to sepsis-induced dysfunction and mitochondrial damage, which is suggested as a major cause of cell metabolism disorders in these patients. The changes affect the metabolism of all macronutrients. The metabolism of all macronutrients is altered. A characteristic change in carbohydrate metabolism is the intensification of glycolysis, which in combination with the failure of entering pyruvate to the tricarboxylic acid cycle increases the formation of lactate. Sepsis also affects lipid metabolism-lipolysis in adipose tissue is upregulated, which leads to an increase in the level of fatty acids and triglycerides in the blood. At the same time, their use is disturbed, which may result in the accumulation of lipids and their toxic metabolites. Changes in the metabolism of ketone bodies and amino acids have also been described. Metabolic disorders in sepsis are an important area of research, both for their potential role as a target for future therapies (metabolic resuscitation) and for optimizing the current treatment, such as clinical nutrition.
Keywords: critical illness; intensive care; metabolic disorders; metabolism; mitochondria; sepsis; septic shock.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Sepsis Care Pathway 2019.Qatar Med J. 2019 Nov 7;2019(2):4. doi: 10.5339/qmj.2019.qccc.4. eCollection 2019. Qatar Med J. 2019. PMID: 31763206 Free PMC article.
-
A murine model of acute and prolonged abdominal sepsis, supported by intensive care, reveals time-dependent metabolic alterations in the heart.Intensive Care Med Exp. 2025 Jan 17;13(1):6. doi: 10.1186/s40635-025-00715-1. Intensive Care Med Exp. 2025. PMID: 39821755 Free PMC article.
-
Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones.Crit Care. 2019 Jul 1;23(1):236. doi: 10.1186/s13054-019-2506-6. Crit Care. 2019. PMID: 31262340 Free PMC article.
-
Sepsis as a Pan-Endocrine Illness-Endocrine Disorders in Septic Patients.J Clin Med. 2021 May 12;10(10):2075. doi: 10.3390/jcm10102075. J Clin Med. 2021. PMID: 34066289 Free PMC article. Review.
-
Changing Definitions of Sepsis.Turk J Anaesthesiol Reanim. 2017 Jun;45(3):129-138. doi: 10.5152/TJAR.2017.93753. Epub 2017 Feb 1. Turk J Anaesthesiol Reanim. 2017. PMID: 28752002 Free PMC article. Review.
Cited by
-
New Perspectives on Circulating Ferritin: Its Role in Health and Disease.Molecules. 2023 Nov 22;28(23):7707. doi: 10.3390/molecules28237707. Molecules. 2023. PMID: 38067440 Free PMC article. Review.
-
U-shaped association between serum triglyceride levels and mortality among septic patients: An analysis based on the MIMIC-IV database.PLoS One. 2023 Nov 27;18(11):e0294779. doi: 10.1371/journal.pone.0294779. eCollection 2023. PLoS One. 2023. PMID: 38011086 Free PMC article.
-
Changes of Serum Pyruvate Kinase M2 Level in Patients with Sepsis and Its Clinical Value.Infect Drug Resist. 2023 Sep 28;16:6437-6449. doi: 10.2147/IDR.S429314. eCollection 2023. Infect Drug Resist. 2023. PMID: 37795205 Free PMC article.
-
A New Strategy for Targeting UCP2 to Modulate Glycolytic Reprogramming as a Treatment for Sepsis A New Strategy for Targeting UCP2.Inflammation. 2024 Oct;47(5):1634-1647. doi: 10.1007/s10753-024-01998-4. Epub 2024 Mar 2. Inflammation. 2024. PMID: 38429403 Free PMC article. Review.
-
On the Intensity of the Microvascular Magnetic Field in Normal State and Septic Shock.J Clin Med. 2025 Apr 6;14(7):2496. doi: 10.3390/jcm14072496. J Clin Med. 2025. PMID: 40217945 Free PMC article.
References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Carré J., Singer M., Moncada S. Nitric Oxide. In: Abraham E., Singer M., editors. Mechanisms of Sepsis-Induced Organ Dysfunction and Recovery. Springer; Berlin/Heidelberg, Germany: New York, NY, USA: 2007. pp. 77–95.
Publication types
LinkOut - more resources
Full Text Sources

