Heat Priming of Lentil (Lens culinaris Medik.) Seeds and Foliar Treatment with γ-Aminobutyric Acid (GABA), Confers Protection to Reproductive Function and Yield Traits under High-Temperature Stress Environments
- PMID: 34072403
- PMCID: PMC8197853
- DOI: 10.3390/ijms22115825
Heat Priming of Lentil (Lens culinaris Medik.) Seeds and Foliar Treatment with γ-Aminobutyric Acid (GABA), Confers Protection to Reproductive Function and Yield Traits under High-Temperature Stress Environments
Abstract
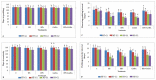
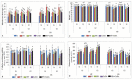
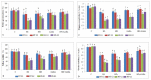
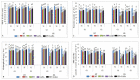
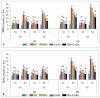
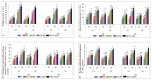
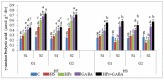
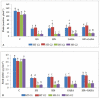
Gradually increasing temperatures at global and local scales are causing heat stress for cool and summer-season food legumes, such as lentil (Lens culinaris Medik.), which is highly susceptible to heat stress, especially during its reproductive stages of development. Hence, suitable strategies are needed to develop heat tolerance in this legume. In the present study, we tested the effectiveness of heat priming (HPr; 6 h at 35 °C) the lentil seeds and a foliar treatment of γ-aminobutyric acid (GABA; 1 mM; applied twice at different times), singly or in combination (HPr+GABA), under heat stress (32/20 °C) in two heat-tolerant (HT; IG2507, IG3263) and two heat-sensitive (HS; IG2821, IG2849) genotypes to mitigate heat stress. The three treatments significantly reduced heat injury to leaves and flowers, particularly when applied in combination, including leaf damage assessed as membrane injury, cellular oxidizing ability, leaf water status, and stomatal conductance. The combined HPr+GABA treatment significantly improved the photosynthetic function, measured as photosynthetic efficiency, chlorophyll concentration, and sucrose synthesis; and significantly reduced the oxidative damage, which was associated with a marked up-regulation in the activities of enzymatic antioxidants. The combined treatment also facilitated the synthesis of osmolytes, such as proline and glycine betaine, by upregulating the expression of their biosynthesizing enzymes (pyrroline-5-carboxylate synthase; betaine aldehyde dehydrogenase) under heat stress. The HPr+GABA treatment caused a considerable enhancement in endogenous levels of GABA in leaves, more so in the two heat-sensitive genotypes. The reproductive function, measured as germination and viability of pollen grains, receptivity of stigma, and viability of ovules, was significantly improved with combined treatment, resulting in enhanced pod number (21-23% in HT and 35-38% in HS genotypes, compared to heat stress alone) and seed yield per plant (22-24% in HT and 37-40% in HS genotypes, in comparison to heat stress alone). The combined treatment (HPr+GABA) was more effective and pronounced in heat-sensitive than heat-tolerant genotypes for all the traits tested. This study offers a potential solution for tackling and protecting heat stress injury in lentil plants.
Keywords: high temperature; legumes; lentil; pods; pollen; seeds; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Siebert S., Ewert F. Future crop production threatened by extreme heat. Environ. Res. Lett. 2014;9:1–4. doi: 10.1088/1748-9326/9/4/041001. - DOI
-
- Prasad P.V.V., Bheemanahalli R., Jagadish S.K. Field crops and the fear of heat stress-opportunities, challenges and future directions. Field Crops Res. 2017;200:114–121. doi: 10.1016/j.fcr.2016.09.024. - DOI
-
- Kaushal N., Bhandari K., Siddique K.H., Nayyar H. Food crops face rising temperatures: An overview of responses, adaptive mechanisms, and approaches to improve heat tolerance. Cogent Food Agric. 2016;2:1–42. doi: 10.1080/23311932.2015.1134380. - DOI
-
- Kaur R., Bains T.S., Bindumadhava H., Nayyar H. Responses of mungbean (Vigna radiata L.) genotypes to heat stress: Effects on reproductive biology, leaf function and yield traits. Sci. Hortic. 2015;197:527–541. doi: 10.1016/j.scienta.2015.10.015. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

