Response of Bovine Cumulus-Oocytes Complexes to Energy Pathway Inhibition during In Vitro Maturation
- PMID: 34072406
- PMCID: PMC8228821
- DOI: 10.3390/genes12060838
Response of Bovine Cumulus-Oocytes Complexes to Energy Pathway Inhibition during In Vitro Maturation
Abstract
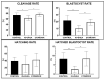
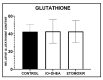
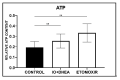
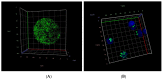
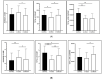
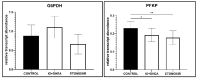
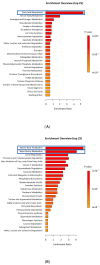
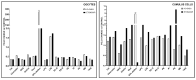
Glucose or fatty acids (FAs) metabolisms may alter the ovarian follicle environment and thus determine oocyte and the nascent embryo quality. The aim of the experiment was to investigate the effect of selective inhibition of glucose (iodoacetate + DHEA) or FA (etomoxir) metabolism on in vitro maturation (IVM) of bovine COCs (cumulus-oocyte complexes) to investigate oocyte's development, quality, and energy metabolism. After in vitro fertilization, embryos were cultured to the blastocyst stage. Lipid droplets, metabolome, and lipidome were analyzed in oocytes and cumulus cells. mRNA expression of the selected genes was measured in the cumulus cells. ATP and glutathione relative levels were measured in oocytes. Changes in FA content in the maturation medium were evaluated by mass spectrometry. Our results indicate that only glucose metabolism is substantial to the oocyte during IVM since only glucose inhibition decreased embryo culture efficiency. The most noteworthy differences in the reaction to the applied inhibition systems were observed in cumulus cells. The upregulation of ketone body metabolism in the cumulus cells of the glucose inhibition group suggest possibly failed attempts of cells to switch into lipid consumption. On the contrary, etomoxir treatment of the oocytes did not affect embryo development, probably due to undisturbed metabolism in cumulus cells. Therefore, we suggest that the energy pathways analyzed in this experiment are not interchangeable alternatives in bovine COCs.
Keywords: cumulus cells; energy metabolism; fatty acids; glucose; oocyte.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Manimaran A., Kumaresan A., Jeyakumar S., Mohanty T.K., Sejian V., Kumar N., Sreela L., Prakash M.A., Mooventhan P., Anantharaj A., et al. Potential of Acute Phase Proteins as Predictor of Postpartum Uterine Infections During Transition Period and Its Regulatory Mechanism in Dairy Cattle. Vet. World. 2016;9:91–100. doi: 10.14202/vetworld.2016.91-100. - DOI - PMC - PubMed
-
- Leroy J.L., Valckx S.D., Jordaens L., De Bie J., Desmet K.L., Van Hoeck V., Britt J.H., Marei W.F., Bols P.E. Nutrition and Maternal Metabolic Health in Relation to Oocyte and Embryo Quality: Critical Views on What We Learned from the Dairy Cow Model. Reprod. Fertil. Dev. 2015;27:693–703. doi: 10.1071/RD14363. - DOI - PubMed
-
- Aardema H., Lolicato F., van de Lest C.H., Brouwers J.F., Vaandrager A.B., van Tol H.T., Roelen B.A., Vos P.L., Helms J.B., Gadella B.M. Bovine Cumulus Cells Protect Maturing Oocytes from Increased Fatty Acid Levels by Massive Intracellular Lipid Storage. Biol. Reprod. 2013;88:164. doi: 10.1095/biolreprod.112.106062. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

