Are BET Inhibitors yet Promising Latency-Reversing Agents for HIV-1 Reactivation in AIDS Therapy?
- PMID: 34072421
- PMCID: PMC8228869
- DOI: 10.3390/v13061026
Are BET Inhibitors yet Promising Latency-Reversing Agents for HIV-1 Reactivation in AIDS Therapy?
Abstract
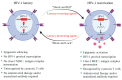
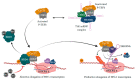
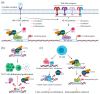
AIDS first emerged decades ago; however, its cure, i.e., eliminating all virus sources, is still unachievable. A critical burden of AIDS therapy is the evasive nature of HIV-1 in face of host immune responses, the so-called "latency." Recently, a promising approach, the "Shock and Kill" strategy, was proposed to eliminate latently HIV-1-infected cell reservoirs. The "Shock and Kill" concept involves two crucial steps: HIV-1 reactivation from its latency stage using a latency-reversing agent (LRA) followed by host immune responses to destroy HIV-1-infected cells in combination with reinforced antiretroviral therapy to kill the progeny virus. Hence, a key challenge is to search for optimal LRAs. Looking at epigenetics of HIV-1 infection, researchers proved that some bromodomains and extra-terminal motif protein inhibitors (BETis) are able to reactivate HIV-1 from latency. However, to date, only a few BETis have shown HIV-1-reactivating functions, and none of them have yet been approved for clinical trial. In this review, we aim to demonstrate the epigenetic roles of BETis in HIV-1 infection and HIV-1-related immune responses. Possible future applications of BETis and their HIV-1-reactivating properties are summarized and discussed.
Keywords: BET protein; BETi; BRD2; BRD4; HIV-1; LRA; epigenetics; immune response; latency-reversing agent; latently HIV-1-infected cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ait-Ammar A., Kula A., Darcis G., Verdikt R., de Wit S., Gautier V., Mallon P.W.G., Marcello A., Rohr O., van Lint C. Current status of latency reversing agents facing the heterogeneity of HIV-1 cellular and tissue reservoirs. Front. Microbiol. 2020;10:3060. doi: 10.3389/fmicb.2019.03060. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

