The M35 Metalloprotease Effector FocM35_1 Is Required for Full Virulence of Fusarium oxysporum f. sp. cubense Tropical Race 4
- PMID: 34072465
- PMCID: PMC8226822
- DOI: 10.3390/pathogens10060670
The M35 Metalloprotease Effector FocM35_1 Is Required for Full Virulence of Fusarium oxysporum f. sp. cubense Tropical Race 4
Abstract
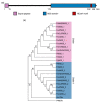
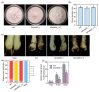
Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4) causes Fusarium wilt of banana, the most devastating disease on a banana plant. The genome of Foc TR4 encodes many candidate effector proteins. However, little is known about the functions of these effector proteins on their contributions to disease development and Foc TR4 virulence. Here, we discovered a secreted metalloprotease, FocM35_1, which is an essential virulence effector of Foc TR4. FocM35_1 was highly upregulated during the early stages of Foc TR4 infection progress in bananas. The FocM35_1 knockout mutant compromised the virulence of Foc TR4. FocM35_1 could interact with the banana chitinase MaChiA, and it decreased banana chitinase activity. FocM35_1 induced cell death in Nicotiana benthamiana while suppressing the INF1-induced hypersensitive response (HR), and its predicted enzymatic site was required for lesion formation and the suppression to INF1-induced HR on N. benthamiana leaves. Importantly, treatment of banana leaves with recombinant FocM35_1 accelerates Foc TR4 infection. Collectively, our study provides evidence that metalloprotease effector FocM35 seems to contribute to pathogen virulence by inhibiting the host immunity.
Keywords: Fusarium wilt; banana; effector; metalloprotease; virulence.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ploetz R.C. Panama disease: An old nemesis rears its ugly head part 2: The Cavendish era and beyond. Apsnet Feature Artic. 2005 doi: 10.1094/APSnetFeature-2005-1005. - DOI
-
- García-Bastidas F.A., Quintero-Vargas J.C., Ayala-Vasquez M., Schermer T., Seidl M.F., Santos-Paiva M., Noguera A.M., Aguilera-Galvez C., Wittenberg A., Hofstede R., et al. First report of Fusarium wilt tropical race 4 in cavendish bananas caused by Fusarium odoratissimum in Colombia. Plant Dis. 2020;104:994. doi: 10.1094/PDIS-09-19-1922-PDN. - DOI
-
- Molina A., Chao C.-P., Dusunceli F., Beed F., Rose L.J., Bothma S., Pretorius A., Vaz A., Mondjana A., Amugoli O.M., et al. Occurrence and spread of the banana fungus Fusarium oxysporum f. sp. cubense TR4 in Mozambique. S. Afr. J. Sci. 2020;116 doi: 10.17159/sajs.2020/8608. - DOI
-
- Viljoen A., Ma L.-J., Molina A.B. Emerging Plant Diseases and Global Food Security. APS press; St. Paul, MN, USA: 2020. CHAPTER 8: Fusarium Wilt (Panama Disease) and Monoculture in Banana Production: Resurgence of a Century-Old Disease; pp. 159–184. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

