Role Played by Receptors for Advanced Glycosylation End Products in Corneal Endothelial Cells after HSV-1 Infection
- PMID: 34072468
- PMCID: PMC8199122
- DOI: 10.3390/ijms22115833
Role Played by Receptors for Advanced Glycosylation End Products in Corneal Endothelial Cells after HSV-1 Infection
Abstract
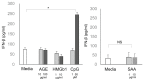
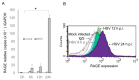
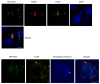
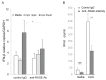
Senescence, sterile inflammation, and infection cause dysfunction of corneal endothelial cells, leading to visual morbidity that may require corneal transplantation. With increasing age, the extracellular matrix is modified by non-enzymatic glycation forming advanced glycation end products (AGEs). The modifications are primarily sensed by the receptors for the AGEs (RAGE) and are manifested as a type I interferon response. Interestingly, in our study, human corneal endothelial cells (HCEn) cells did not respond to the typical RAGE ligands, including the AGEs, high mobility group box 1 (HMGB1), and serum amyloid-A (SAA). Instead, HCEn cells responded exclusively to the CpG DNA, which is possessed by typical corneal pathogen, herpes simplex virus-1 (HSV-1). Upon HSV-1 infection, the surface expression of RAGE was increased, and endocytosed HSV-1 was associated with RAGE and CpG DNA receptor, TLR9. RAGE DNA transfection markedly increased interferon-β secretion by CpG DNA or HSV-1 infection. HSV-1 infection-induced interferon-β secretion was abolished by TLR9 inhibition and partially by RAGE inhibition. Global transcriptional response analysis confirmed that RAGE and TLR9 were both significantly involved in type I interferon responses. We conclude that RAGE is a sensor of HSV-1 infection and provokes a type I interferon response.
Keywords: advanced glycation end products; corneal endothelial cell; herpes simplex virus; receptor for advanced glycosylation end products; toll-like receptor 9.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Herpes simplex virus type 1-induced transcriptional networks of corneal endothelial cells indicate antigen presentation function.Invest Ophthalmol Vis Sci. 2011 Jun 16;52(7):4282-93. doi: 10.1167/iovs.10-6911. Invest Ophthalmol Vis Sci. 2011. PMID: 21540477
-
Indoleamine 2,3-dioxygenase 1 in corneal endothelial cells limits herpes simplex virus type 1-induced acquired immune response.Br J Ophthalmol. 2015 Oct;99(10):1435-42. doi: 10.1136/bjophthalmol-2015-306863. Epub 2015 Jul 3. Br J Ophthalmol. 2015. PMID: 26142400
-
Role of interferon regulatory factor 7 in corneal endothelial cells after HSV-1 infection.Sci Rep. 2021 Aug 13;11(1):16487. doi: 10.1038/s41598-021-95823-9. Sci Rep. 2021. PMID: 34389779 Free PMC article.
-
Glycation reaction and the role of the receptor for advanced glycation end-products in immunity and social behavior.Glycoconj J. 2021 Jun;38(3):303-310. doi: 10.1007/s10719-020-09956-6. Epub 2020 Oct 27. Glycoconj J. 2021. PMID: 33108607 Review.
-
Receptor for Advanced Glycation End Products (RAGE) in Type 1 Diabetes Pathogenesis.Curr Diab Rep. 2016 Oct;16(10):100. doi: 10.1007/s11892-016-0782-y. Curr Diab Rep. 2016. PMID: 27612847 Review.
Cited by
-
Pathological role of RAGE underlying progression of various diseases: its potential as biomarker and therapeutic target.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr;398(4):3467-3487. doi: 10.1007/s00210-024-03595-6. Epub 2024 Nov 26. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39589529 Review.
-
Herpes simplex keratitis: A brief clinical overview.World J Virol. 2024 Mar 25;13(1):89934. doi: 10.5501/wjv.v13.i1.89934. World J Virol. 2024. PMID: 38616855 Free PMC article. Review.
References
-
- Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

