Biological Potential of Polyethylene Glycol (PEG)-Functionalized Graphene Quantum Dots in In Vitro Neural Stem/Progenitor Cells
- PMID: 34072613
- PMCID: PMC8226482
- DOI: 10.3390/nano11061446
Biological Potential of Polyethylene Glycol (PEG)-Functionalized Graphene Quantum Dots in In Vitro Neural Stem/Progenitor Cells
Abstract
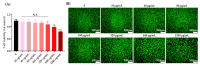
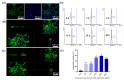
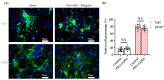
Stem cell therapy is one of the novel and prospective fields. The ability of stem cells to differentiate into different lineages makes them attractive candidates for several therapies. It is essential to understand the cell fate, distribution, and function of transplanted cells in the local microenvironment before their applications. Therefore, it is necessary to develop an accurate and reliable labeling method of stem cells for imaging techniques to track their translocation after transplantation. The graphitic quantum dots (GQDs) are selected among various stem cell labeling and tracking strategies which have high photoluminescence ability, photostability, relatively low cytotoxicity, tunable surface functional groups, and delivering capacity. Since GQDs interact easily with the cell and interfere with cell behavior through surface functional groups, an appropriate surface modification needs to be considered to get close to the ideal labeling nanoprobes. In this study, polyethylene glycol (PEG) is used to improve biocompatibility while simultaneously maintaining the photoluminescent potentials of GQDs. The biochemically inert PEG successfully covered the surface of GQDs. The PEG-GQDs composites show adequate bioimaging capabilities when internalized into neural stem/progenitor cells (NSPCs). Furthermore, the bio-inertness of the PEG-GQDs is confirmed. Herein, we introduce the PEG-GQDs as a valuable tool for stem cell labeling and tracking for biomedical therapies in the field of neural regeneration.
Keywords: biocompatibility; cytotoxicity; neural stem/progenitor cells (NSPCs); polyethylene glycol functionalized-graphene quantum dots (PEG-GQDs); the visible bio labeling system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Highly luminescent polyethylene glycol-passivated graphene quantum dots for light emitting diodes.RSC Adv. 2020 Jul 22;10(46):27418-27423. doi: 10.1039/d0ra02257h. eCollection 2020 Jul 21. RSC Adv. 2020. PMID: 35516959 Free PMC article.
-
Planted Graphene Quantum Dots for Targeted, Enhanced Tumor Imaging and Long-Term Visualization of Local Pharmacokinetics.Adv Mater. 2023 Apr;35(15):e2210809. doi: 10.1002/adma.202210809. Epub 2023 Mar 3. Adv Mater. 2023. PMID: 36740642 Free PMC article.
-
Synthesis of strongly green-photoluminescent graphene quantum dots for drug carrier.Colloids Surf B Biointerfaces. 2013 Dec 1;112:192-6. doi: 10.1016/j.colsurfb.2013.07.025. Epub 2013 Jul 19. Colloids Surf B Biointerfaces. 2013. PMID: 23974005
-
A holistic review on red fluorescent graphene quantum dots, its synthesis, unique properties with emphasis on biomedical applications.Heliyon. 2024 Aug 8;10(16):e35760. doi: 10.1016/j.heliyon.2024.e35760. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220916 Free PMC article. Review.
-
Recent Advances in the Cancer Bioimaging with Graphene Quantum Dots.Curr Med Chem. 2018;25(25):2876-2893. doi: 10.2174/0929867324666170223154145. Curr Med Chem. 2018. PMID: 28240167 Review.
Cited by
-
Recent Advances in Monitoring Stem Cell Status and Differentiation Using Nano-Biosensing Technologies.Nanomaterials (Basel). 2022 Aug 25;12(17):2934. doi: 10.3390/nano12172934. Nanomaterials (Basel). 2022. PMID: 36079970 Free PMC article. Review.
-
Progress in spinal cord organoid research: advancing understanding of neural development, disease modelling, and regenerative medicine.Biomater Transl. 2024 Nov 15;5(4):355-371. doi: 10.12336/biomatertransl.2024.04.003. eCollection 2024. Biomater Transl. 2024. PMID: 39872925 Free PMC article. Review.
-
Is Graphene Shortening the Path toward Spinal Cord Regeneration?ACS Nano. 2022 Sep 27;16(9):13430-13467. doi: 10.1021/acsnano.2c04756. Epub 2022 Aug 24. ACS Nano. 2022. PMID: 36000717 Free PMC article. Review.
-
Biodistribution of Intravenously Transplanted Mitochondria Conjugated with Graphene Quantum Dots in Diabetic Rats.J Fluoresc. 2024 Nov;34(6):2725-2735. doi: 10.1007/s10895-023-03480-0. Epub 2023 Oct 28. J Fluoresc. 2024. PMID: 37897517
-
Spinal cord injury: molecular mechanisms and therapeutic interventions.Signal Transduct Target Ther. 2023 Jun 26;8(1):245. doi: 10.1038/s41392-023-01477-6. Signal Transduct Target Ther. 2023. PMID: 37357239 Free PMC article. Review.
References
-
- Feigin V.L., Nichols E., Alam T., Bannick M.S., Beghi E., Blake N., Culpepper W.J., Dorsey E.R., Elbaz A., Ellenbogen R.G., et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

