Interventions and Outcomes for Neoadjuvant Treatment of T4 Colon Cancer: A Scoping Review
- PMID: 34072615
- PMCID: PMC8261638
- DOI: 10.3390/curroncol28030191
Interventions and Outcomes for Neoadjuvant Treatment of T4 Colon Cancer: A Scoping Review
Abstract
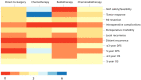
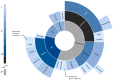
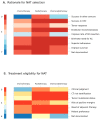
While adjuvant treatment of colon cancers that penetrate the serosa (T4) have been well-established, neoadjuvant strategies have yet to be formally evaluated. Our objective was to perform a scoping review of eligibility criteria, treatment regimens, and primary outcomes for neoadjuvant approaches to T4 colon cancer. A librarian-led, systematic search of MEDLINE, Embase, Cochrane Library, Web of Science, and CINAHL up to 11 February 2020 was performed. Primary research evaluating neoadjuvant treatment in T4 colon cancer were included. Screening and data abstraction were performed in duplicate; analyses were descriptive or thematic. A total of twenty studies were included, most of which were single-arm, single-center, and retrospective. The primary objectives of the literature to date has been to evaluate treatment feasibility, tumor response, disease-free survival, and overall survival in healthy patients. Conventional XELOX and FOLFOX chemotherapy were the most commonly administered interventions. Rationale for selecting a specific regimen and for treatment eligibility criteria were poorly documented across studies. The current literature on neoadjuvant strategies for T4 colon cancer is overrepresented by single-center, retrospective studies that evaluate treatment feasibility and efficacy in healthy patients. Future studies should prioritize evaluating clear selection criteria and rationale for specific neoadjuvant strategies. Validation of outcomes in multi-center, randomized trials for XELOX and FOLFOX have the most to contribute to the growing evidence for this poorly managed disease.
Keywords: T4 colon cancer; chemoradiotherapy; chemotherapy; locally advanced colon cancer; neoadjuvant therapy; radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Neoadjuvant therapy versus direct to surgery for T4 colon cancer: meta-analysis.Br J Surg. 2021 Dec 17;109(1):30-36. doi: 10.1093/bjs/znab382. Br J Surg. 2021. PMID: 34921604
-
Capecitabine for hormone receptor-positive versus hormone receptor-negative breast cancer.Cochrane Database Syst Rev. 2021 May 26;5(5):CD011220. doi: 10.1002/14651858.CD011220.pub2. Cochrane Database Syst Rev. 2021. PMID: 34037241 Free PMC article.
-
Neoadjuvant FOLFOX 4 versus FOLFOX 4 with Cetuximab versus immediate surgery for high-risk stage II and III colon cancers: a multicentre randomised controlled phase II trial--the PRODIGE 22--ECKINOXE trial.BMC Cancer. 2015 Jul 10;15:511. doi: 10.1186/s12885-015-1507-3. BMC Cancer. 2015. PMID: 26156156 Free PMC article. Clinical Trial.
-
Neoadjuvant chemotherapy in locally advanced colon cancer: A systematic review with proportional meta-analysis.Eur J Surg Oncol. 2025 Mar;51(3):109560. doi: 10.1016/j.ejso.2024.109560. Epub 2024 Dec 20. Eur J Surg Oncol. 2025. PMID: 39869958
-
Survival and safety after neoadjuvant chemotherapy or upfront surgery for locally advanced colon cancer: meta-analysis.Br J Surg. 2024 Jan 31;111(2):znae021. doi: 10.1093/bjs/znae021. Br J Surg. 2024. PMID: 38381934 Free PMC article.
Cited by
-
Effective Delivery of siRNA-Loaded Nanoparticles for Overcoming Oxaliplatin Resistance in Colorectal Cancer.Front Oncol. 2022 Feb 21;12:827891. doi: 10.3389/fonc.2022.827891. eCollection 2022. Front Oncol. 2022. PMID: 35265524 Free PMC article.
-
Minimally Invasive Surgery: Is It a Risk Factor for Postoperative Peritoneal Metastasis in pT4 Colon Cancer?Ann Surg Oncol. 2025 Jan;32(1):158-164. doi: 10.1245/s10434-024-16177-w. Epub 2024 Sep 16. Ann Surg Oncol. 2025. PMID: 39283578
-
Predicting treatment failure in stage III colon cancer patients after radical surgery.Front Oncol. 2024 May 16;14:1397468. doi: 10.3389/fonc.2024.1397468. eCollection 2024. Front Oncol. 2024. PMID: 38817900 Free PMC article.
-
PD-1 blockade enhances the effect of targeted chemotherapy on locally advanced pMMR/MSS colorectal cancer.Cancer Med. 2024 Jun;13(12):e7224. doi: 10.1002/cam4.7224. Cancer Med. 2024. PMID: 38888366 Free PMC article.
-
Colorectal cancer: a comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments.Cancer Metastasis Rev. 2024 Jun;43(2):729-753. doi: 10.1007/s10555-023-10158-3. Epub 2023 Dec 19. Cancer Metastasis Rev. 2024. PMID: 38112903 Review.
References
-
- Klaver C.E.L., Gietelink L., Bemelman W.A., Wouters M.W.J.M., Wiggers T., Tollenaar R.A.E.M., Tanis P.J., on behalf of the Dutch Surgical Colorectal Audit group Locally Advanced Colon Cancer: Evaluation of Current Clinical Practice and Treatment Outcomes at the Population Level. J. Natl. Compr. Cancer Netw. 2017;15:181–190. doi: 10.6004/jnccn.2017.0019. - DOI - PubMed
-
- Nakafusa Y., Tanaka T., Tanaka M., Kitajima Y., Sato S., Miyazaki K. Comparison of Multivisceral Resection and Standard Operation for Locally Advanced Colorectal Cancer: Analysis of Prognostic Factors for Short-Term and Long-Term Outcome. Dis. Colon Rectum. 2004;47:2055–2063. doi: 10.1007/s10350-004-0716-7. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

