Glucose Metabolic Dysfunction in Neurodegenerative Diseases-New Mechanistic Insights and the Potential of Hypoxia as a Prospective Therapy Targeting Metabolic Reprogramming
- PMID: 34072616
- PMCID: PMC8198281
- DOI: 10.3390/ijms22115887
Glucose Metabolic Dysfunction in Neurodegenerative Diseases-New Mechanistic Insights and the Potential of Hypoxia as a Prospective Therapy Targeting Metabolic Reprogramming
Abstract
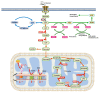
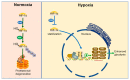
Glucose is the main circulating energy substrate for the adult brain. Owing to the high energy demand of nerve cells, glucose is actively oxidized to produce ATP and has a synergistic effect with mitochondria in metabolic pathways. The dysfunction of glucose metabolism inevitably disturbs the normal functioning of neurons, which is widely observed in neurodegenerative disease. Understanding the mechanisms of metabolic adaptation during disease progression has become a major focus of research, and interventions in these processes may relieve the neurons from degenerative stress. In this review, we highlight evidence of mitochondrial dysfunction, decreased glucose uptake, and diminished glucose metabolism in different neurodegeneration models such as Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington's disease (HD). We also discuss how hypoxia, a metabolic reprogramming strategy linked to glucose metabolism in tumor cells and normal brain cells, and summarize the evidence for hypoxia as a putative therapy for general neurodegenerative disease.
Keywords: brain energy metabolism; glucose; hypoxia; metabolic reprogramming; neurodegenerative disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Oxidative Stress: A Key Modulator in Neurodegenerative Diseases.Molecules. 2019 Apr 22;24(8):1583. doi: 10.3390/molecules24081583. Molecules. 2019. PMID: 31013638 Free PMC article. Review.
-
Insights into therapeutic approaches for the treatment of neurodegenerative diseases targeting metabolic syndrome.Mol Biol Rep. 2025 Feb 21;52(1):260. doi: 10.1007/s11033-025-10346-0. Mol Biol Rep. 2025. PMID: 39982557 Review.
-
Reactive Oxygen Species and Their Impact in Neurodegenerative Diseases: Literature Landscape Analysis.Antioxid Redox Signal. 2021 Feb 10;34(5):402-420. doi: 10.1089/ars.2019.7952. Epub 2020 Mar 9. Antioxid Redox Signal. 2021. PMID: 32030995 Review.
-
Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia.J Neurol Sci. 2005 Jun 15;233(1-2):145-62. doi: 10.1016/j.jns.2005.03.012. J Neurol Sci. 2005. PMID: 15896810 Review.
-
Mitochondrial dynamics, a key executioner in neurodegenerative diseases.Mitochondrion. 2019 Jul;47:151-173. doi: 10.1016/j.mito.2018.11.002. Epub 2018 Nov 5. Mitochondrion. 2019. PMID: 30408594 Review.
Cited by
-
Overcoming mitochondrial dysfunction in neurodegenerative diseases.Neural Regen Res. 2023 Jul;18(7):1486-1488. doi: 10.4103/1673-5374.360279. Neural Regen Res. 2023. PMID: 36571346 Free PMC article. No abstract available.
-
Exploring the Role of Glycolytic Enzymes PFKFB3 and GAPDH in the Modulation of Aβ and Neurodegeneration and Their Potential of Therapeutic Targets in Alzheimer's Disease.Appl Biochem Biotechnol. 2023 Jul;195(7):4673-4688. doi: 10.1007/s12010-023-04340-0. Epub 2023 Jan 24. Appl Biochem Biotechnol. 2023. PMID: 36692648 Review.
-
Molecular Pathophysiological Mechanisms in Huntington's Disease.Biomedicines. 2022 Jun 17;10(6):1432. doi: 10.3390/biomedicines10061432. Biomedicines. 2022. PMID: 35740453 Free PMC article. Review.
-
SARS-CoV-2 Causes Lung Inflammation through Metabolic Reprogramming and RAGE.Viruses. 2022 May 6;14(5):983. doi: 10.3390/v14050983. Viruses. 2022. PMID: 35632725 Free PMC article.
-
Cross talk mechanisms of aerobic exercise training on obesity, type 2 diabetes, and Alzheimer's disease: the role of insulin resistance.Rev Assoc Med Bras (1992). 2022 Jul;68(7):963-967. doi: 10.1590/1806-9282.20211210. Rev Assoc Med Bras (1992). 2022. PMID: 35946775 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

