Neural Plasticity in the Brain during Neuropathic Pain
- PMID: 34072638
- PMCID: PMC8228570
- DOI: 10.3390/biomedicines9060624
Neural Plasticity in the Brain during Neuropathic Pain
Abstract
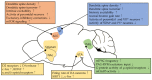
Neuropathic pain is an intractable chronic pain, caused by damage to the somatosensory nervous system. To date, treatment for neuropathic pain has limited effects. For the development of efficient therapeutic methods, it is essential to fully understand the pathological mechanisms of neuropathic pain. Besides abnormal sensitization in the periphery and spinal cord, accumulating evidence suggests that neural plasticity in the brain is also critical for the development and maintenance of this pain. Recent technological advances in the measurement and manipulation of neuronal activity allow us to understand maladaptive plastic changes in the brain during neuropathic pain more precisely and modulate brain activity to reverse pain states at the preclinical and clinical levels. In this review paper, we discuss the current understanding of pathological neural plasticity in the four pain-related brain areas: the primary somatosensory cortex, the anterior cingulate cortex, the periaqueductal gray, and the basal ganglia. We also discuss potential treatments for neuropathic pain based on the modulation of neural plasticity in these brain areas.
Keywords: anterior cingulate cortex; basal ganglia; neural plasticity; neuropathic pain; periaqueductal grey; primary somatosensory cortex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Brainstem Pain-Modulation Circuitry and Its Plasticity in Neuropathic Pain: Insights From Human Brain Imaging Investigations.Front Pain Res (Lausanne). 2021 Jul 30;2:705345. doi: 10.3389/fpain.2021.705345. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295481 Free PMC article. Review.
-
Neural markers of neuropathic pain associated with maladaptive plasticity in spinal cord injury.Pain Pract. 2015 Apr;15(4):371-7. doi: 10.1111/papr.12237. Epub 2014 Aug 28. Pain Pract. 2015. PMID: 25169195 Review.
-
Neural circuit remodeling and structural plasticity in the cortex during chronic pain.Korean J Physiol Pharmacol. 2016 Jan;20(1):1-8. doi: 10.4196/kjpp.2016.20.1.1. Epub 2015 Dec 31. Korean J Physiol Pharmacol. 2016. PMID: 26807017 Free PMC article. Review.
-
Deep brain stimulation of the dorsal anterior cingulate cortex for the treatment of chronic neuropathic pain.Neurosurg Focus. 2015 Jun;38(6):E11. doi: 10.3171/2015.3.FOCUS1543. Neurosurg Focus. 2015. PMID: 26030699 Review.
-
Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex.J Neurochem. 2017 May;141(4):486-498. doi: 10.1111/jnc.14001. Epub 2017 Mar 27. J Neurochem. 2017. PMID: 28251660 Review.
Cited by
-
The Impact of P-Glycoprotein on Opioid Analgesics: What's the Real Meaning in Pain Management and Palliative Care?Int J Mol Sci. 2022 Nov 16;23(22):14125. doi: 10.3390/ijms232214125. Int J Mol Sci. 2022. PMID: 36430602 Free PMC article. Review.
-
Modulation of Neuropathic Pain by Glial Regulation in the Insular Cortex of Rats.Front Mol Neurosci. 2022 Apr 13;15:815945. doi: 10.3389/fnmol.2022.815945. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35493331 Free PMC article.
-
Unraveling the Potential of Electroanalgesia: A Literature Review of Current Therapeutics.Cureus. 2024 May 26;16(5):e61122. doi: 10.7759/cureus.61122. eCollection 2024 May. Cureus. 2024. PMID: 38919207 Free PMC article. Review.
-
Increased mGluR5 in somatostatin-positive interneurons mediates deactivation of the mPFC in a mouse model of neuropathic pain.Exp Mol Med. 2025 Apr;57(4):888-899. doi: 10.1038/s12276-025-01435-y. Epub 2025 Apr 10. Exp Mol Med. 2025. PMID: 40211051 Free PMC article.
-
Metabotropic Glutamate Receptor 5 in the Dysgranular Zone of Primary Somatosensory Cortex Mediates Neuropathic Pain in Rats.Biomedicines. 2022 Jul 7;10(7):1633. doi: 10.3390/biomedicines10071633. Biomedicines. 2022. PMID: 35884938 Free PMC article.
References
-
- Raja S.N., Carr D.B., Cohen M., Finnerup N.B., Flor H., Gibson S., Keefe F.J., Mogil J.S., Ringkamp M., Sluka K.A., et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain. 2020;161:1976–1982. doi: 10.1097/j.pain.0000000000001939. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources