Dexamethasone Downregulates Autophagy through Accelerated Turn-Over of the Ulk-1 Complex in a Trabecular Meshwork Cells Strain: Insights on Steroid-Induced Glaucoma Pathogenesis
- PMID: 34072647
- PMCID: PMC8198647
- DOI: 10.3390/ijms22115891
Dexamethasone Downregulates Autophagy through Accelerated Turn-Over of the Ulk-1 Complex in a Trabecular Meshwork Cells Strain: Insights on Steroid-Induced Glaucoma Pathogenesis
Abstract
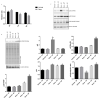
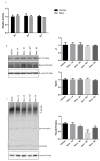
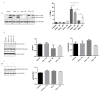
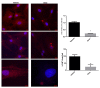
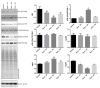
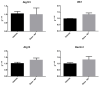
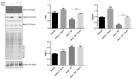
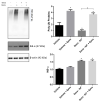
Steroid-induced glaucoma is a severe pathological condition, sustained by a rapidly progressive increase in intraocular pressure (IOP), which is diagnosed in a subset of subjects who adhere to a glucocorticoid (GC)-based therapy. Molecular and clinical studies suggest that either natural or synthetic GCs induce a severe metabolic dysregulation of Trabecular Meshwork Cells (TMCs), an endothelial-derived histotype with phagocytic and secretive functions which lay at the iridocorneal angle in the anterior segment of the eye. Since TMCs physiologically regulate the composition and architecture of trabecular meshwork (TM), which is the main outflow pathway of aqueous humor, a fluid which shapes the eye globe and nourishes the lining cell types, GCs are supposed to trigger a pathological remodeling of the TM, inducing an IOP increase and retina mechanical compression. The metabolic dysregulation of TMCs induced by GCs exposure has never been characterized at the molecular detail. Herein, we report that, upon dexamethasone exposure, a TMCs strain develops a marked inhibition of the autophagosome biogenesis pathway through an enhanced turnover of two members of the Ulk-1 complex, the main platform for autophagy induction, through the Ubiquitin Proteasome System (UPS).
Keywords: autophagy; glaucoma; glucocorticoids; intraocular pressure; trabecular meshwork; ubiquitin proteasome system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Delayed Turnover of Proteasome Processing of Myocilin upon Dexamethasone Stimulation Introduces the Profiling of Trabecular Meshwork Cells' Ubiquitylome.Int J Mol Sci. 2024 Sep 17;25(18):10017. doi: 10.3390/ijms251810017. Int J Mol Sci. 2024. PMID: 39337505 Free PMC article.
-
Effects of thailanstatins on glucocorticoid response in trabecular meshwork and steroid-induced glaucoma.Invest Ophthalmol Vis Sci. 2013 May 3;54(5):3137-42. doi: 10.1167/iovs.12-11480. Invest Ophthalmol Vis Sci. 2013. PMID: 23548621 Free PMC article.
-
Mitochondria and Autophagy Dysfunction in Glucocorticoid-Induced Ocular Hypertension/Glaucoma Mice Model.Curr Eye Res. 2020 Feb;45(2):190-198. doi: 10.1080/02713683.2019.1657462. Epub 2019 Aug 29. Curr Eye Res. 2020. PMID: 31425668
-
The trabecular meshwork: Structure, function and clinical implications. A review of the literature.J Fr Ophtalmol. 2020 Sep;43(7):e217-e230. doi: 10.1016/j.jfo.2020.05.002. Epub 2020 Jun 16. J Fr Ophtalmol. 2020. PMID: 32561029 Review.
-
Trabecular meshwork as a new target for the treatment of glaucoma.Drug News Perspect. 2006 Apr;19(3):151-8. doi: 10.1358/dnp.2006.19.3.985929. Drug News Perspect. 2006. PMID: 16804567 Review.
Cited by
-
Dexamethasone Induces Senescence-Associated Changes in Trabecular Meshwork Cells by Increasing ROS Levels Via the TGFβ/Smad3-NOX4 Axis.Cell Transplant. 2023 Jan-Dec;32:9636897231177356. doi: 10.1177/09636897231177356. Cell Transplant. 2023. PMID: 37265069 Free PMC article.
-
The Delayed Turnover of Proteasome Processing of Myocilin upon Dexamethasone Stimulation Introduces the Profiling of Trabecular Meshwork Cells' Ubiquitylome.Int J Mol Sci. 2024 Sep 17;25(18):10017. doi: 10.3390/ijms251810017. Int J Mol Sci. 2024. PMID: 39337505 Free PMC article.
-
The interplay of autophagy and oxidative stress in the pathogenesis and therapy of retinal degenerative diseases.Cell Biosci. 2022 Jan 3;12(1):1. doi: 10.1186/s13578-021-00736-9. Cell Biosci. 2022. PMID: 34980273 Free PMC article. Review.
-
AUTOPHAGY IN THE EYE: FROM PHYSIOLOGY TO PATHOPHYSOLOGY.Autophagy Rep. 2023;2(1):2178996. doi: 10.1080/27694127.2023.2178996. Epub 2023 Mar 1. Autophagy Rep. 2023. PMID: 37034386 Free PMC article.
-
Comparison of postoperative cyclosporine 2.0% versus betamethasone 0.1% eye drops following trabeculectomy: A randomized clinical trial.Indian J Ophthalmol. 2025 Mar 1;73(Suppl 2):S272-S281. doi: 10.4103/IJO.IJO_345_24. Epub 2024 Oct 25. Indian J Ophthalmol. 2025. PMID: 39446816 Free PMC article. Clinical Trial.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

