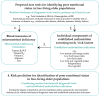
Predicting Malnutrition Risk with Data from Routinely Measured Clinical Biochemical Diagnostic Tests in Free-Living Older Populations
- PMID: 34072686
- PMCID: PMC8226876
- DOI: 10.3390/nu13061883
Predicting Malnutrition Risk with Data from Routinely Measured Clinical Biochemical Diagnostic Tests in Free-Living Older Populations
Abstract
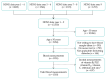
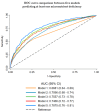
Malnutrition (undernutrition) in older adults is often not diagnosed before its adverse consequences have occurred, despite the existence of established screening tools. As a potential method of early detection, we examined whether readily available and routinely measured clinical biochemical diagnostic test data could predict poor nutritional status. We combined 2008-2017 data of 1518 free-living individuals ≥50 years from the United Kingdom National Diet and Nutrition Survey (NDNS) and used logistic regression to determine associations between routine biochemical diagnostic test data, micronutrient deficiency biomarkers, and established malnutrition indicators (components of screening tools) in a three-step validation process. A prediction model was created to determine how effectively routine biochemical diagnostic tests and established malnutrition indicators predicted poor nutritional status (defined by ≥1 micronutrient deficiency in blood of vitamins B6, B12 and C; selenium; or zinc). Significant predictors of poor nutritional status were low concentrations of total cholesterol, haemoglobin, HbA1c, ferritin and vitamin D status, and high concentrations of C-reactive protein; except for HbA1c, these were also associated with established malnutrition indicators. Additional validation was provided by the significant association of established malnutrition indicators (low protein, fruit/vegetable and fluid intake) with biochemically defined poor nutritional status. The prediction model (including biochemical tests, established malnutrition indicators and covariates) showed an AUC of 0.79 (95% CI: 0.76-0.81), sensitivity of 66.0% and specificity of 78.1%. Clinical routine biochemical diagnostic test data have the potential to facilitate early detection of malnutrition risk in free-living older populations. However, further validation in different settings and against established malnutrition screening tools is warranted.
Keywords: biochemical diagnostic tests; micronutrient deficiency biomarker; screening tool; undernutrition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

