Bovine Parainfluenza Virus Type 3 (BPIV3) Enters HeLa Cells via Clathrin-Mediated Endocytosis in a Cholesterol- and Dynamin-Dependent Manner
- PMID: 34072688
- PMCID: PMC8228847
- DOI: 10.3390/v13061035
Bovine Parainfluenza Virus Type 3 (BPIV3) Enters HeLa Cells via Clathrin-Mediated Endocytosis in a Cholesterol- and Dynamin-Dependent Manner
Abstract
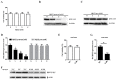
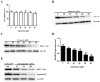
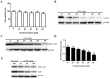
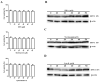
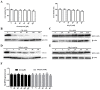
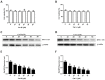
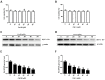
Bovine parainfluenza virus 3 (BPIV3) is a crucial causative agent of respiratory disease in young and adult cattle. No specific therapies are available for BPIV3 infection. Understanding the internalization pathway of the virus will provide a new strategy for the development of antiviral therapy. Here, the mechanism of BPIV3 entry into HeLa cells was analyzed using RNA silencing and pharmacological inhibitors. Treatment of HeLa cells with hypertonic medium prevented BPIV3 internalization. These results indicated that BPIV3 entered HeLa cells via receptor-mediated endocytosis. Moreover, removing cell membrane cholesterol through MβCD treatment hampered viral penetration but not viral replication. In addition, BPIV3 infection was inhibited by pretreatment with dynasore or chlorpromazine (CPZ) or knockdown of dynamin II or clathrin heavy chain. However, virus entry was unaffected by nystatin, EIPA, wortmannin, or cytochalasin D treatment or caveolin-1 knockdown. These data demonstrated that the entry of BPIV3 into HeLa cells was dependent on clathrin-mediated endocytosis but not on caveolae-mediated endocytosis or the macropinocytosis pathway. Many viruses are transported to endosomes, which provide an acidic environment and release their genome upon separation from primary endocytic vesicles. However, we found that BPIV3 infection required endosomal cathepsins, but not a low pH. In summary, we show, for the first time, that BPIV3 enters HeLa cells through the clathrin-mediated endocytosis pathway, presenting novel insights into the invasion mechanism of Paramyxoviridae.
Keywords: BPIV3; cathepsins; clathrin; endocytosis; virus entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Entry of bovine parainfluenza virus type 3 into MDBK cells occurs via clathrin-mediated endocytosis and macropinocytosis in a acid-dependent manner.Vet Microbiol. 2021 Aug;259:109148. doi: 10.1016/j.vetmic.2021.109148. Epub 2021 Jun 15. Vet Microbiol. 2021. PMID: 34147763
-
Entry of Classical Swine Fever Virus into PK-15 Cells via a pH-, Dynamin-, and Cholesterol-Dependent, Clathrin-Mediated Endocytic Pathway That Requires Rab5 and Rab7.J Virol. 2016 Sep 29;90(20):9194-208. doi: 10.1128/JVI.00688-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489278 Free PMC article.
-
Entry of Challenge Virus Standard (CVS) -11 into N2a cells via a clathrin-mediated, cholesterol-, dynamin-, pH-dependent endocytic pathway.Virol J. 2019 Jun 13;16(1):80. doi: 10.1186/s12985-019-1186-9. Virol J. 2019. PMID: 31196105 Free PMC article.
-
Involvement of adaptor proteins in clathrin-mediated endocytosis of virus entry.Microb Pathog. 2021 Dec;161(Pt A):105278. doi: 10.1016/j.micpath.2021.105278. Epub 2021 Nov 2. Microb Pathog. 2021. PMID: 34740810 Review.
-
Revisiting Pathogen Exploitation of Clathrin-Independent Endocytosis: Mechanisms and Implications.Cells. 2025 May 16;14(10):731. doi: 10.3390/cells14100731. Cells. 2025. PMID: 40422234 Free PMC article. Review.
Cited by
-
Temporal Dynamics of the Ruminant Type I IFN-Induced Antiviral State against Homologous Parainfluenza Virus 3 Challenge In Vitro.Viruses. 2022 May 11;14(5):1025. doi: 10.3390/v14051025. Viruses. 2022. PMID: 35632770 Free PMC article.
-
Revealing the Cell Entry Dynamic Mechanism of Single Rabies Virus Particle.Chem Res Chin Univ. 2022;38(3):838-842. doi: 10.1007/s40242-022-2069-y. Epub 2022 May 2. Chem Res Chin Univ. 2022. PMID: 35530119 Free PMC article.
-
Schisandra chinensis inhibits the entry of BoHV-1 by blocking PI3K-Akt pathway and enhances the m6A methylation of gD to inhibit the entry of progeny virus.Front Microbiol. 2024 Jul 22;15:1444414. doi: 10.3389/fmicb.2024.1444414. eCollection 2024. Front Microbiol. 2024. PMID: 39104584 Free PMC article.
-
Critical role of G3BP1 in bovine parainfluenza virus type 3 (BPIV3)-inhibition of stress granules formation and viral replication.Front Immunol. 2024 Apr 16;15:1358036. doi: 10.3389/fimmu.2024.1358036. eCollection 2024. Front Immunol. 2024. PMID: 38690262 Free PMC article.
-
LINC08148 promotes the caveola-mediated endocytosis of Zika virus through upregulating transcription of Src.J Virol. 2024 Jun 13;98(6):e0170523. doi: 10.1128/jvi.01705-23. Epub 2024 May 14. J Virol. 2024. PMID: 38742902 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

