Taurine Augments Telomerase Activity and Promotes Chondrogenesis in Dental Pulp Stem Cells
- PMID: 34072707
- PMCID: PMC8228366
- DOI: 10.3390/jpm11060491
Taurine Augments Telomerase Activity and Promotes Chondrogenesis in Dental Pulp Stem Cells
Abstract
Background: Stem cell therapy has become an advanced and state-of-the-art procedure to regenerate lost tissues of the human body. Cartilage repair is a challenging task in which stem cells find potential application. One of the important biologic modifiers that can cause chondrogenic differentiation of stem cells is taurine. However, taurine has not been investigated for its effects on dental pulp derived stem cell (DPSC) chondrogenic differentiation.
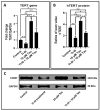
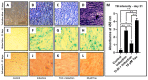
Objective: The objective of the study was to investigate if taurine administration to DPSCs heralds chondrogenic differentiation as ascertained by expression of SOX9, COL2A1, ACAN, ELN, and COMP. The study also investigated if the differentiated cells synthesized glycosaminoglycans, a marker of cartilage formation. The study also aimed to assess proliferative activity of the cells after taurine administration by measuring the hTERT gene and protein expression.
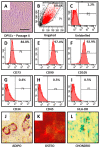
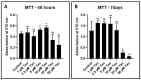
Materials and methods: DPSCs were obtained from a molecular biology laboratory and characterization of stem cell markers was done by flow cytometry. The cells were subjected to a MTT assay using various concentrations of taurine. Following this, hTERT gene and protein estimation was done in the control, telomerase inhibitor treated DPSC (TI-III), 10 μM taurine treated DPSC, and TI-III + 10 μM taurine treated DPSCs. A polymerase chain reaction was done to assess gene expression of SOX9, COL2A1, ACAN, ELN, and COMP genes and glycosaminoglycans were estimated in control cells, Induced DPSCs, induced and TI-III treated DPSCs, and 10 μM taurine treated DPSCs.
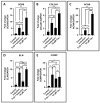
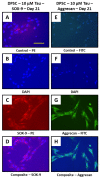
Results: DPSCs expressed CD73, CD90, and CD105 and did not express CD34, CD45, and HLA-DR, which demonstrated that they were mesenchymal stem cells. The MTT assay revealed that various concentrations of taurine did not affect the cell viability of DPSCs. A concentration of 10 μM of taurine was used for further assays. With regard to the hTERT gene and protein expression, the taurine treated cells expressed the highest levels that were statistically significant compared to the other groups. Taurine was also found to restore hTERT expression in telomerase inhibitor treated cells. With regard to chondrogenesis related genes, taurine administration significantly increased the expression of SOX9, COL2A1, ACAN, and ELN genes in DPSCs and caused a significant increase in glycosaminoglycan production by the cells.
Conclusions: Taurine can be regarded a biologic modifier that can significantly augment chondrogenic differentiation of DPSCs and can find potential applications in regenerative medicine in the area of cartilage regeneration.
Keywords: chondrogenesis; dental pulp; mesenchymal stem cells; regenerative medicine; taurine; telomerase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Differential SOD2 and GSTZ1 profiles contribute to contrasting dental pulp stem cell susceptibilities to oxidative damage and premature senescence.Stem Cell Res Ther. 2021 Feb 17;12(1):142. doi: 10.1186/s13287-021-02209-9. Stem Cell Res Ther. 2021. PMID: 33596998 Free PMC article.
-
Preliminary Evaluation of Proliferation, Wound Healing Properties, Osteogenic and Chondrogenic Potential of Dental Pulp Stem Cells Obtained from Healthy and Periodontitis Affected Teeth.Cells. 2021 Aug 18;10(8):2118. doi: 10.3390/cells10082118. Cells. 2021. PMID: 34440887 Free PMC article.
-
Multilineage Differentiation Potential of Human Dental Pulp Stem Cells-Impact of 3D and Hypoxic Environment on Osteogenesis In Vitro.Int J Mol Sci. 2020 Aug 26;21(17):6172. doi: 10.3390/ijms21176172. Int J Mol Sci. 2020. PMID: 32859105 Free PMC article.
-
Systematic Review of Human Dental Pulp Stem Cells for Cartilage Regeneration.Tissue Eng Part B Rev. 2020 Feb;26(1):1-12. doi: 10.1089/ten.TEB.2019.0140. Epub 2020 Jan 22. Tissue Eng Part B Rev. 2020. PMID: 31744404
-
Dental Pulp Stem Cells: Advances to Applications.Stem Cells Cloning. 2020 Feb 13;13:33-42. doi: 10.2147/SCCAA.S166759. eCollection 2020. Stem Cells Cloning. 2020. PMID: 32104005 Free PMC article. Review.
Cited by
-
Ectopic Activation of Fgf8 in Dental Mesenchyme Causes Incisor Agenesis and Molar Microdontia.Int J Mol Sci. 2024 Jun 27;25(13):7045. doi: 10.3390/ijms25137045. Int J Mol Sci. 2024. PMID: 39000154 Free PMC article.
-
In Silico Novel Drug Design Targeting the Oral Microbiome: Endodontic and Periodontal Pathogenic Bacteria.Microorganisms. 2021 Nov 22;9(11):2400. doi: 10.3390/microorganisms9112400. Microorganisms. 2021. PMID: 34835525 Free PMC article.
-
Antimicrobial and Cytotoxic Activity of Ocimum tenuiflorum and Stevia rebaudiana-Mediated Silver Nanoparticles - An In vitro Study.Contemp Clin Dent. 2023 Apr-Jun;14(2):109-114. doi: 10.4103/ccd.ccd_369_21. Epub 2022 Nov 11. Contemp Clin Dent. 2023. PMID: 37547431 Free PMC article.
-
Strategies to Convert Cells into Hyaline Cartilage: Magic Spells for Adult Stem Cells.Int J Mol Sci. 2022 Sep 22;23(19):11169. doi: 10.3390/ijms231911169. Int J Mol Sci. 2022. PMID: 36232468 Free PMC article. Review.
-
Functional Role of Taurine in Aging and Cardiovascular Health: An Updated Overview.Nutrients. 2023 Sep 30;15(19):4236. doi: 10.3390/nu15194236. Nutrients. 2023. PMID: 37836520 Free PMC article. Review.
References
-
- Bentley G., Biant L.C., Carrington R.W.J., Akmal M., Goldberg A., Williams A.M., Skinner J.A., Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J. Bone Jt. Surg. Br. 2003;85:223–230. doi: 10.1302/0301-620X.85B2.13543. - DOI - PubMed
-
- Knutsen G., Engebretsen L., Ludvigsen T.C., Drogset J.O., Grøntvedt T., Solheim E., Strand T., Roberts S., Isaksen V., Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J. Bone Jt. Surg. Am. 2004;86:455–464. doi: 10.2106/00004623-200403000-00001. - DOI - PubMed
-
- Tatullo M., Spagnuolo G., Codispoti B., Zamparini F., Zhang A., Esposti M., Aparicio C., Rengo C., Nuzzolese M., Manzoli L., et al. PLA-Based Mineral-Doped Scaffolds Seeded with Human Periapical Cyst-Derived MSCs: A Promising Tool for Regenerative Healing in Dentistry. Materials. 2019;12:597. doi: 10.3390/ma12040597. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

