Multifunctional Enkephalin Analogs with a New Biological Profile: MOR/DOR Agonism and KOR Antagonism
- PMID: 34072734
- PMCID: PMC8229567
- DOI: 10.3390/biomedicines9060625
Multifunctional Enkephalin Analogs with a New Biological Profile: MOR/DOR Agonism and KOR Antagonism
Abstract
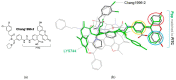
In our previous studies, we developed a series of mixed MOR/DOR agonists that are enkephalin-like tetrapeptide analogs with an N-phenyl-N-piperidin-4-ylpropionamide (Ppp) moiety at the C-terminus. Further SAR study on the analogs, initiated by the findings from off-target screening, resulted in the discovery of LYS744 (6, Dmt-DNle-Gly-Phe(p-Cl)-Ppp), a multifunctional ligand with MOR/DOR agonist and KOR antagonist activity (GTPγS assay: IC50 = 52 nM, Imax = 122% cf. IC50 = 59 nM, Imax = 100% for naloxone) with nanomolar range of binding affinity (Ki = 1.3 nM cf. Ki = 2.4 nM for salvinorin A). Based on its unique biological profile, 6 is considered to possess high therapeutic potential for the treatment of chronic pain by modulating pathological KOR activation while retaining analgesic efficacy attributed to its MOR/DOR agonist activity.
Keywords: analgesic effects; kappa receptor antagonists; multifunctional activity; opioid receptors; peptidomimetics; plasma stability; template-based alignment modeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Anand J.P., Montgomery D. Multifunctional opioid ligands. Handb. Exp. Pharmacol. 2018;247:21–51. - PubMed
-
- Balboni G., Onnis V., Congiu C., Zotti M., Sasaki Y., Ambo A., Bryant S.D., Jinsmaa Y., Lazarus L.H., Lazzari I., et al. Further studies on the effect of lysine at the C-terminus of the Dmt-Tic opioid pharmacophore. Bioorg. Med. Chem. 2007;15:3143–3151. doi: 10.1016/j.bmc.2007.02.039. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

