Enzyme-Loaded Flower-Shaped Nanomaterials: A Versatile Platform with Biosensing, Biocatalytic, and Environmental Promise
- PMID: 34072882
- PMCID: PMC8227841
- DOI: 10.3390/nano11061460
Enzyme-Loaded Flower-Shaped Nanomaterials: A Versatile Platform with Biosensing, Biocatalytic, and Environmental Promise
Abstract
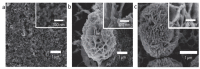
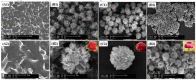
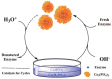
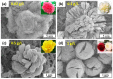
As a result of their unique structural and multifunctional characteristics, organic-inorganic hybrid nanoflowers (hNFs), a newly developed class of flower-like, well-structured and well-oriented materials has gained significant attention. The structural attributes along with the surface-engineered functional entities of hNFs, e.g., their size, shape, surface orientation, structural integrity, stability under reactive environments, enzyme stabilizing capability, and organic-inorganic ratio, all significantly contribute to and determine their applications. Although hNFs are still in their infancy and in the early stage of robust development, the recent hike in biotechnology at large and nanotechnology in particular is making hNFs a versatile platform for constructing enzyme-loaded/immobilized structures for different applications. For instance, detection- and sensing-based applications, environmental- and sustainability-based applications, and biocatalytic and biotransformation applications are of supreme interest. Considering the above points, herein we reviewed current advances in multifunctional hNFs, with particular emphasis on (1) critical factors, (2) different metal/non-metal-based synthesizing processes (i.e., (i) copper-based hNFs, (ii) calcium-based hNFs, (iii) manganese-based hNFs, (iv) zinc-based hNFs, (v) cobalt-based hNFs, (vi) iron-based hNFs, (vii) multi-metal-based hNFs, and (viii) non-metal-based hNFs), and (3) their applications. Moreover, the interfacial mechanism involved in hNF development is also discussed considering the following three critical points: (1) the combination of metal ions and organic matter, (2) petal formation, and (3) the generation of hNFs. In summary, the literature given herein could be used to engineer hNFs for multipurpose applications in the biosensing, biocatalysis, and other environmental sectors.
Keywords: bio-catalysis; biosensing cues; biosynthesis; hybrid nanoflowers; influencing factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Zdarta J., Meyer A.S., Jesionowski T., Pinelo M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts. 2018;8:92. doi: 10.3390/catal8020092. - DOI
-
- Basso A., Serban S. Industrial Applications of Immobilized Enzymes—A Review. Mol. Catal. 2019;479:110607. doi: 10.1016/j.mcat.2019.110607. - DOI
-
- Truppo M.D., Hughes G. Development of an Improved Immobilized CAL-B for the Enzymatic Resolution of a Key Intermediate to Odanacatib. Org. Process Res. Dev. 2011;15:1033–1035. doi: 10.1021/op200157c. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

